 |
 |
 |
| |
RESISTANCE PROFILE OF MK-8507, A NOVEL NNRTI SUITABLE FOR WEEKLY ORAL HIV TREATMENT
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Tracy L . Diamond1, Ming-Tain Lai1, Meizhen Feng1, Min Xu1, Nancy A. Sachs1, Daria Hazuda1, Ernest Asante-Appiah1, Jay A. Grobler1 1Merck & Co, Inc, West Point, PA, USA
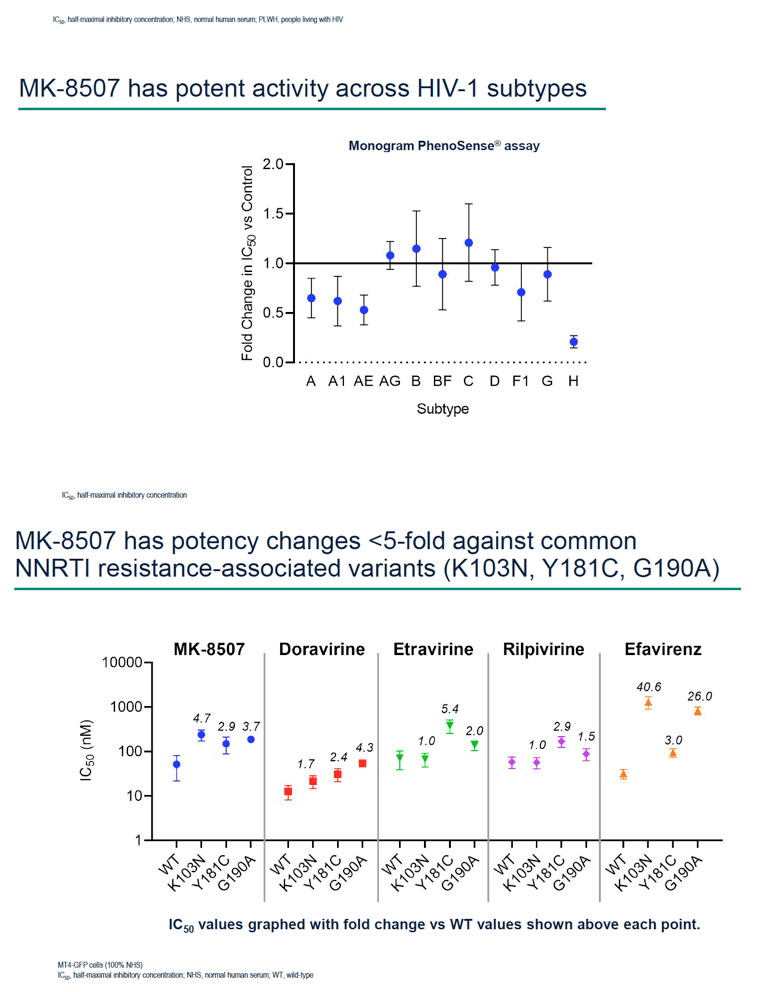
Background: MK-8507 is a novel non-nucleoside reverse transcriptase inhibitor (NNRTI) in clinical development as a once-weekly oral treatment for HIV-1 infection. To support the development of MK-8507, in vitro assays were performed to assess its virologic profile and compare its activity to clinically approved NNRTIs.
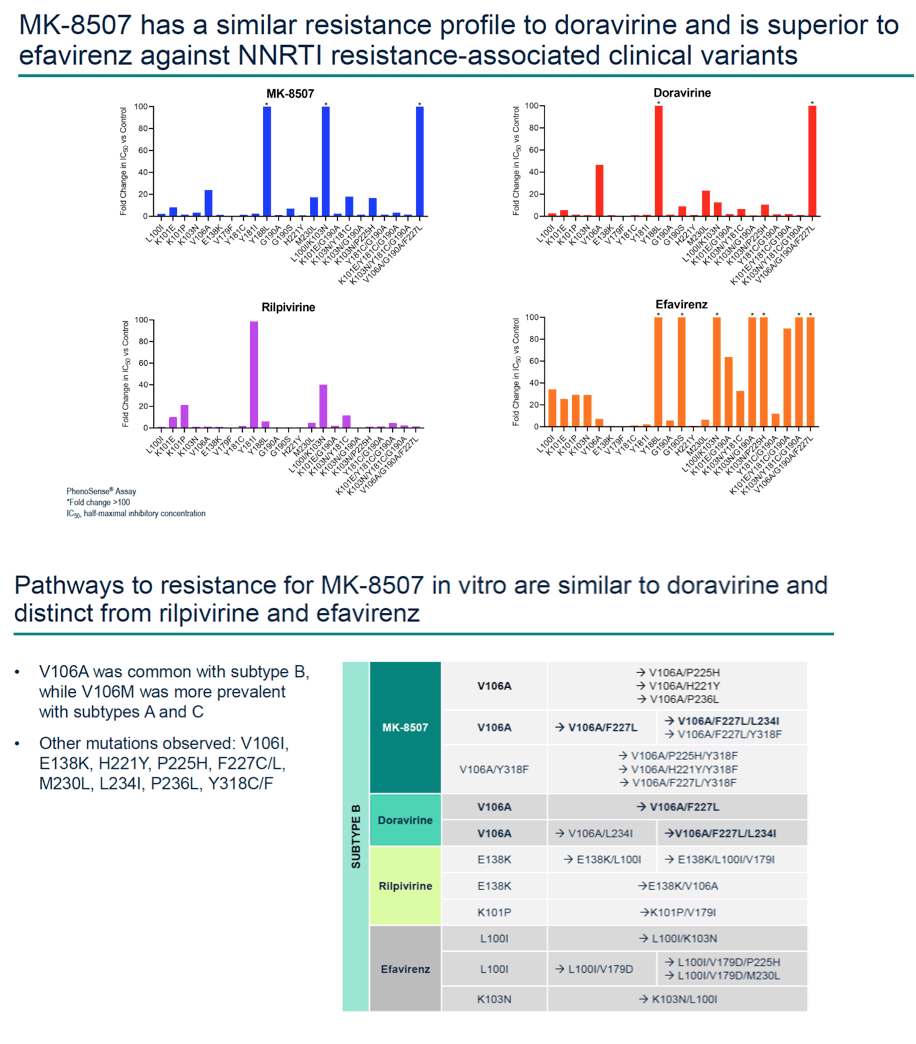
Methods: Half-maximal inhibitory concentration (IC50) of MK-8507 against wild-type subtype B (WT) HIV-1 was assessed in a multiple cycle assay. Activity against clinical variants representing multiple HIV-1 subtypes was determined using the PhenoSense® assay. MK-8507 was evaluated in two-drug combination antiviral and cytotoxicity assays with each of 17 antiviral agents across mechanistic classes. Viral resistance selection studies with escalating concentrations of MK-8507 were conducted on cells infected with HIV-1 subtype A, B, or C to determine pathways to MK-8507 resistance. Antiviral activity of MK-8507 on common NNRTI resistance-associated variants and a panel of variants that emerged under MK-8507 selective pressure was determined in multiple cycle assays. Activity against a panel of clinical NNRTI resistance- associated variants was determined by PhenoSense®.
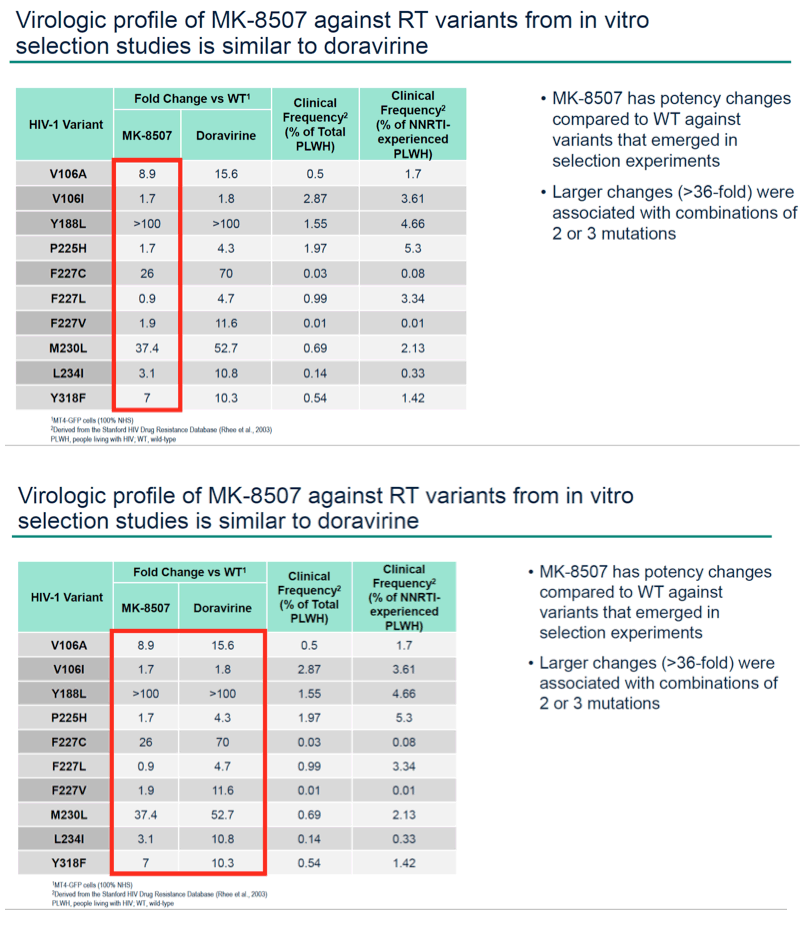
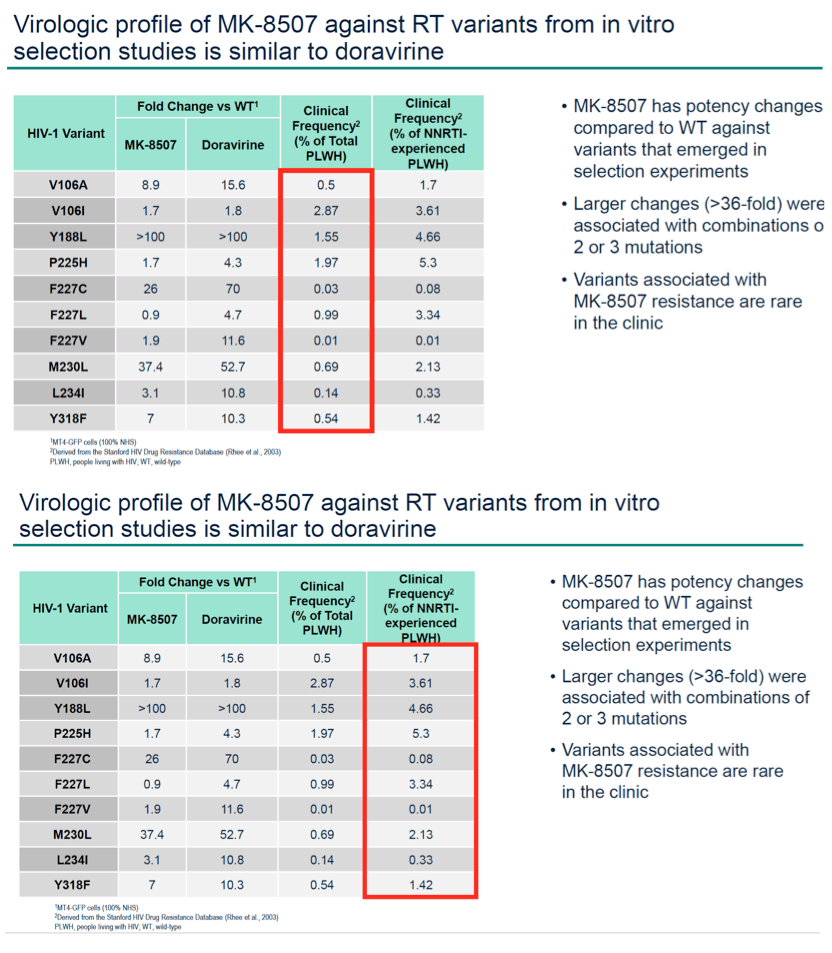
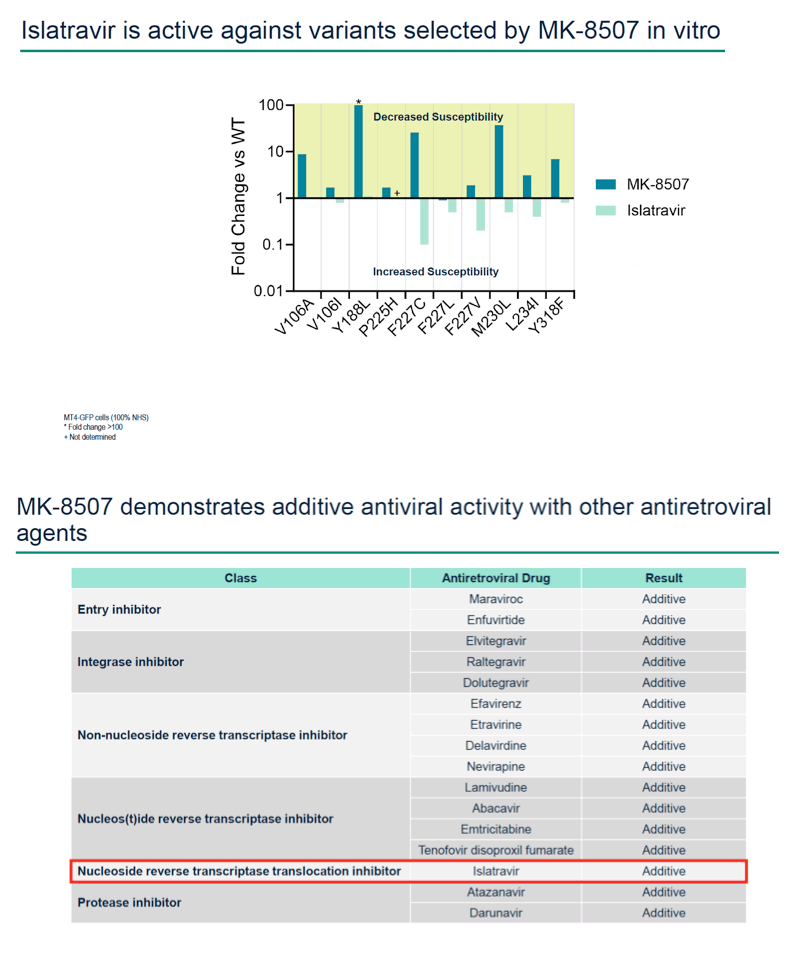
Results: MK-8507 had an IC50 of 51.3 nM against WT HIV-1 and maintained similar activity across HIV-1 subtypes. It displayed additive antiviral activity and was not antagonistic in combination with all antiretrovirals tested, including islatravir. In resistance selection experiments V106A was the primary mutation observed with subtype B virus and V106M was the primary mutation observed with subtypes A and C. Most other mutations observed (E138K, H221Y, Y188L, P225H, F227C/L, m230L, L234I, P236L, Y318F) were in combination with V106A/M mutations. Variants observed in vitro under MK-8507 selective pressure are uncommon in the clinic (prevalence <2% in the Stanford HIV Drug Resistance Database). MK-8507 had potency reductions from 0.9 to 544.0-fold against variants that emerged in selection experiments and had <5 fold-shifts against common NNRTI resistance-associated variants, K103N, Y181C, and G190A. It also maintained <5-fold shift against 13 of 21 clinical NNRTI variants tested in the PhenoSense® assay.
Conclusion: MK-8507 is a novel and potent NNRTI with activity against common NNRTI resistance-associated variants and has antiviral activity and a resistance profile similar to doravirine and distinct from efavirenz. This in vitro profile supports further development of MK-8507 as a component of a once weekly regimen.

|
| |
|
 |
 |
|
|