 |
 |
 |
| |
BAMLANIVIMAB PREVENTS COVID-19 MORBIDITY AND MORTALITY IN NURSING-HOME SETTING
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Myron S. Cohen, Ajay Nirula, Mark Mulligan, Richard Novak, Mary Marovich, Alexander Stemer, Andrew C. Adams, Andrew E. Schade, Jack Knorr, Jay L. Tuttle, Janelle Sabo, Paul Klekotka, Lei Shen, Daniel M. Skovronsky, for the BLAZE-2 Study Team (Lilly/NIAID/CoVPN)
Program Abstract
Myron S . Cohen1, Ajay Nirula2, Mark Mulligan3, Richard Novak4, Mary Marovich5, Alexander Stemer6, Andrew C. Adams2, Andrew E. Schade2, Jack Knorr2, Jay
L. Tuttle2, Janelle Sabo2, Paul Klekotka2, Lei Shen2, Daniel M. Skovronsky2, for BLAZE-2 study team (Lilly/NIAID/CoVPN)
1Institute of Global Health and Infectious Diseases, University of North Carolina
at Chapel Hill, Chapel Hill, NC, USA, 2Eli Lilly and Company, Indianapolis, IN, USA, 3New York University Langone Vaccine Center, Division of Infectious Diseases and Immunology, New York University Grossman School of Medicine, New York, NY,
USA, 4University of Illinois College of Medicine, Chicago, IL, USA, 5Vaccine Research Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA, 6Indiana University School of Medicine, Gary, IN, USA
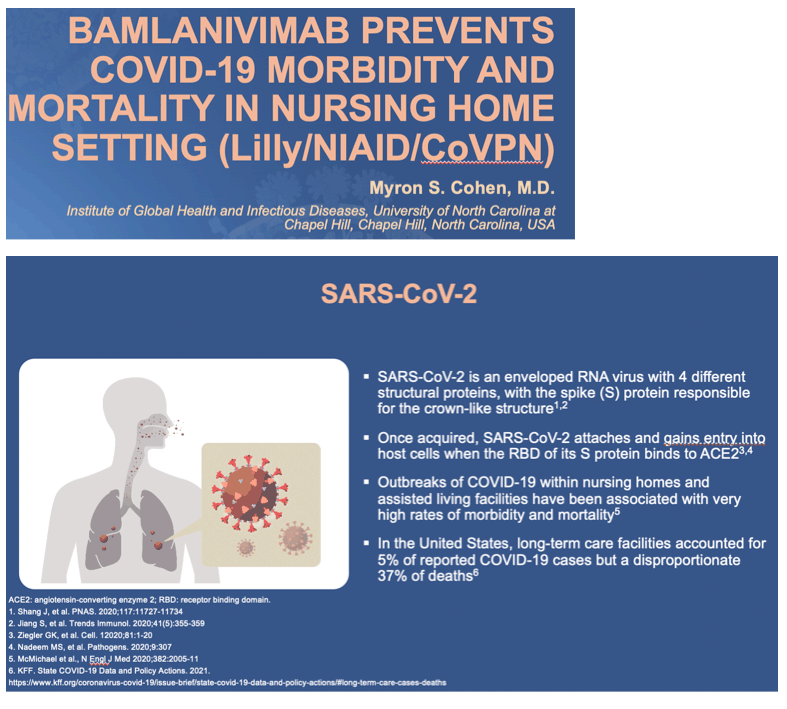
Background: The COVID-19 pandemic has disproportionately affected residents of skilled nursing and assisted living facilities. Interventions are urgently needed to protect this vulnerable population. Bamlanivimab is a potent neutralizing monoclonal antibody that binds the receptor-binding domain of the spike protein of SARS-CoV-2. This study evaluates the safety and efficacy of bamlanivimab in preventing COVID-19.
Methods: BLAZE-2 is a Phase 3, randomized, double-blind, placebo-controlled, single-dose study that enrolled residents and staff at skilled nursing and assisted living facilities reporting at least one confirmed SARS-CoV-2 case. Eligible participants received bamlanivimab (4200 mg) or placebo intravenously. Nasal swabs were collected at baseline and weekly through day 57 to determine SARS-CoV-2 infection status via reverse transcriptase polymerase chain reaction (RT-PCR). COVID-19-releated symptoms and signs were recorded daily. The primary analysis prevention population included participants negative at baseline for SARS-CoV-2 by RT-PCR and serology. The primary endpoint was incidence of mild or worse COVID-19 by day 57.
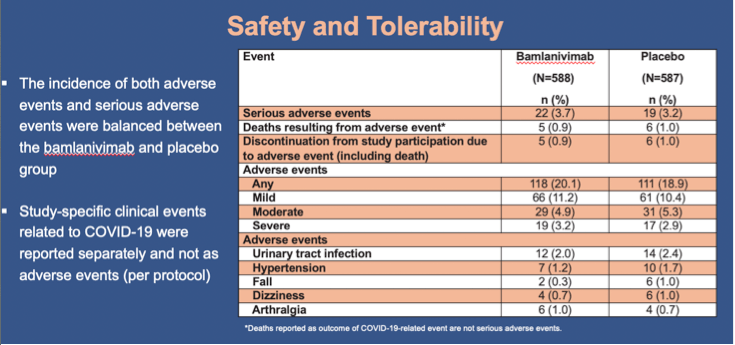
Results: Of the 1175 participants dosed, 966 (82.2%) comprised the prevention population. The prevention population included 299 residents for whom the median age was 76 years (range 31-104), 234 (78.3%) were aged ≥65, and 178 (59.5%) were female. All were considered at high risk for development of severe COVID-19. The proportion of residents in the prevention population with mild or worse COVID-19 by day 57 was significantly lower in the bamlanivimab group compared with the placebo group (odds ratio [OR], 0.20; 95% confidence interval [CI], 0.08 to 0.49; p<0.001)(Figure). For this same group, bamlanivimab was associated with significant reductions in the incidence of moderate or worse COVID-19 by day 57 (OR, 0.20; 95% CI, 0.08 to 0.49; p<0.001) and incident SARS-CoV-2 infection by day 29 (OR, 0.23; CI, 0.11 to 0.48; p<0.001) compared with placebo. Of the 16 deaths reported during the study, all 5 that were attributed to COVID-19 were in the placebo group. The incidence of both adverse events and serious adverse events were balanced between the bamlanivimab and placebo group.
Conclusion: Bamlanivimab was highly effective in reducing the incidence of symptomatic COVID-19 and SARS-CoV-2 infection and was well tolerated. These findings demonstrate the potential beneficial impact of bamlanivimab use on COVID-19 morbidity and mortality among skilled nursing facility residents.


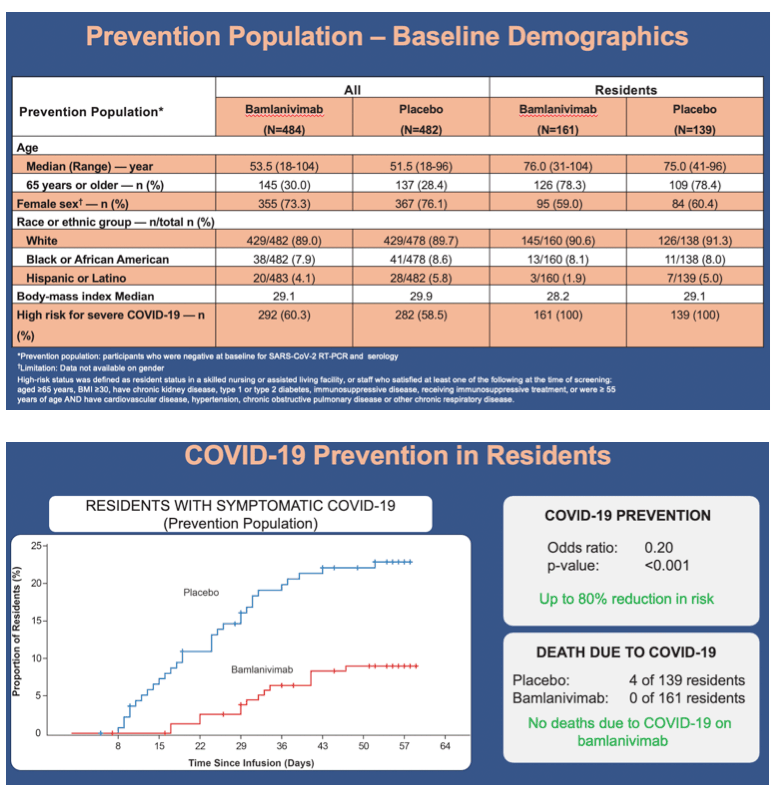
•In the resident prevention population, incidence of mild or worse COVID-19 by day 57 was significantly lower in the bamlanivimab group compared with the placebo group (8.8% vs 22.5%; odds ratio, 0.20; 95% CI, 0.08 to 0.49; p<0.001)
•By day 57, in the prevention population, 4 COVID-19-related deaths occurred in the placebo group versus 0 in the bamlanivimab group
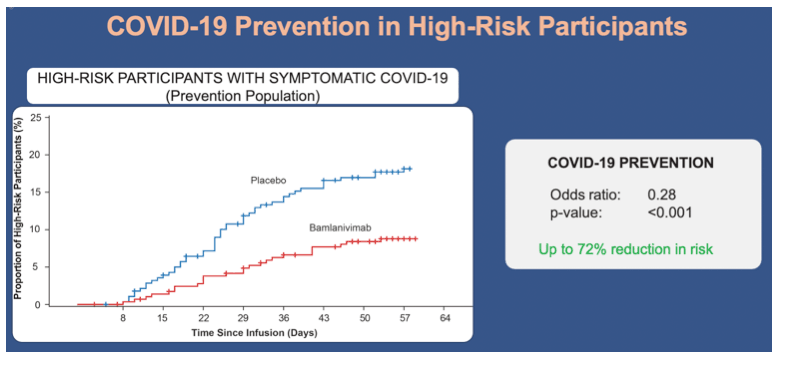
•In the prevention population at high risk for development of severe COVID-19, the incidence of mild or worse COVID-19 was also lower in the bamlanivimab group compared with the placebo group (8.7% vs 17.8%; odds ratio, 0.28; 95% CI, 0.15 to 0.53; p<0.001)
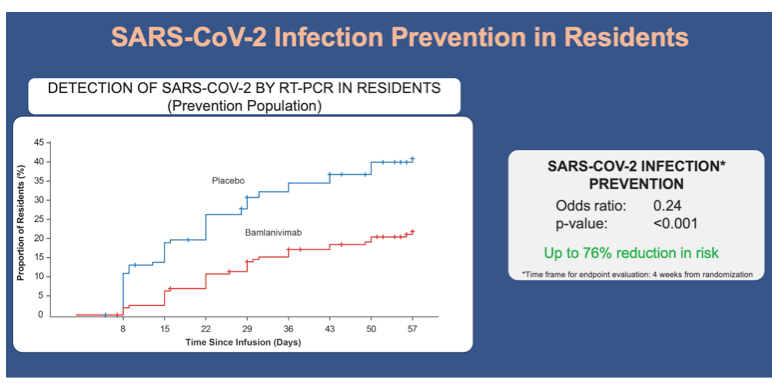
•Bamlanivimab was also associated with significantly lower incidences of SARS-CoV-2 infection by week 4 in the resident subpopulation:
•15.1% of resident participants who received bamlanivimab, and 31.9% of residents who received placebo (odds ratio, 0.24; 95% CI, 0.12 to 0.51)
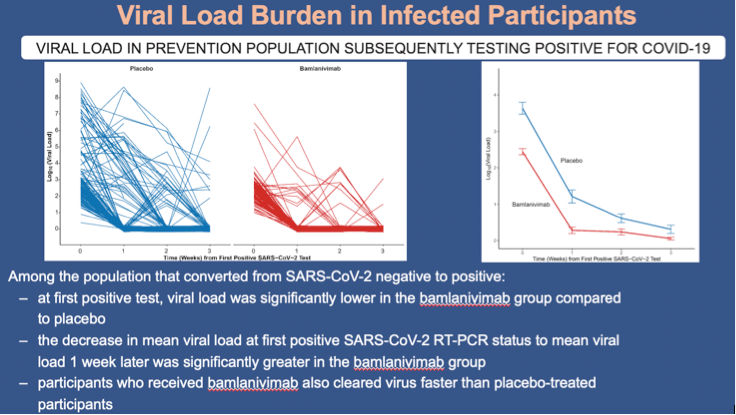
•Among the population that converted from SARS-CoV-2 negative to positive within 8 weeks of randomization:
•Participants who received bamlanivimab had lower viral loads compared with placebo recipients at the time of their first positive test (2.44 vs 3.64 log10 viral load) and the decrease in mean viral load at first positive RT-PCR status to mean viral load at week 1 was significantly greater in the bamlanivimab group compared to the placebo (-0.8 log10 viral load; 95% CI, -1.22 to -0.39; p<0.001)
•participants who received bamlanivimab also cleared virus faster than placebo-treated participants

|
| |
|
 |
 |
|
|