 |
 |
 |
| |
SAFETY/EFFICACY OF DTG VS EFV, TDF VS
TAF IN PREGNANCY/POSTPARTUM: IMPAACT 2010 TRIAL
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Lameck Chinula1, Sean Brummel2, Lauren Ziemba2, Katie McCarthy3, Benjamin Johnston4, Nahida Chakhtoura5, Patrick Jean-Philippe6, Lynda Stranix-Chibanda7,
Violet Korutaro8, Haseena Cassim9, Fairlie Lee10, Gaerolwe Masheto11, Paul Sax12, Judith S. Currier13, Shahin Lockman12
1University of North Carolina Project–Malawi, Lilongwe, Malawi, 2Harvard TH Chan School of Public Health, Boston, MA, USA, 3FHI 360, Durham, NC, USA, 4Frontier Science & Technology Research Foundation, Inc, Amherst, NY, USA, 5National Institute of Child Health and Human Development, Bethesda, MD, USA, 6National Institute of Allergy and Infectious Diseases, Rockville, MD, USA, 7University of Zimbabwe, Harare, Zimbabwe, 8Baylor College of Medicine Children's Foundation, Kampala, Uganda, 9Soweto IMPAACT CRS, Johannesburg, South Africa, 10Wits Reproductive Health and HIV Institute, Johannesburg, South Africa, 11Botswana Harvard AIDS Institute Partnership, Gabarone, Botswana, 12Brigham and Women’s Hospital, Boston, MA, USA, 13University of California Los Angeles, Los Angeles, CA, USA 7 8
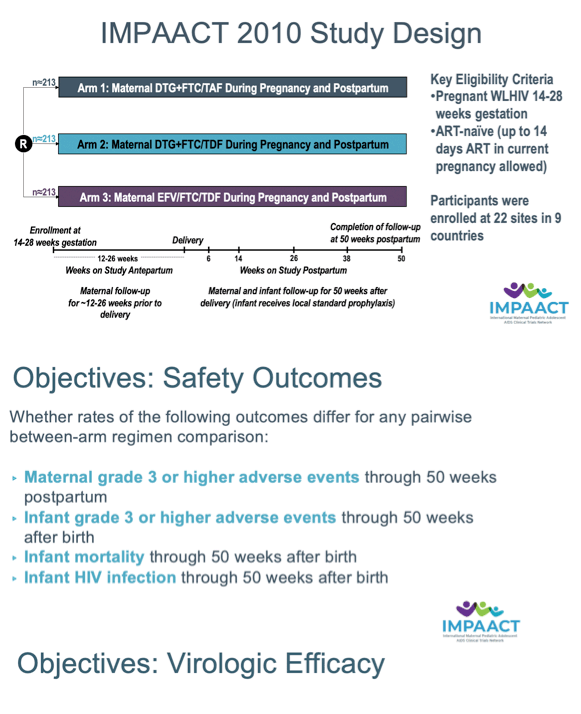
Background: We previously reported safety and virologic efficacy of dolutegravir (DTG)+emtricitabine (FTC)/tenofovir alafenamide fumarate (TAF) vs DTG+FTC/tenofovir disoproxil fumarate (TDF) vs efavirenz (EFV)/FTC/TDF through delivery. We now present results from enrollment through week 50 postpartum (PP).
Methods: Pregnant women with HIV-1 in 9 countries were randomized 1:1:1 to start open-label DTG+FTC/TAF, DTG+FTC/TDF, or EFV/FTC/TDF at 14-28 weeks gestational age (GA). Safety outcomes included pairwise regimen comparisons of grade≥3 maternal and infant adverse events (AEs, primary), infant mortality, and infant HIV infection. Safety probabilities were estimated with the Kaplan-Meier method. Efficacy analyses include comparison of maternal HIV RNA <200cp/mL at week 50 PP between the combined DTG arms and EFV arm.
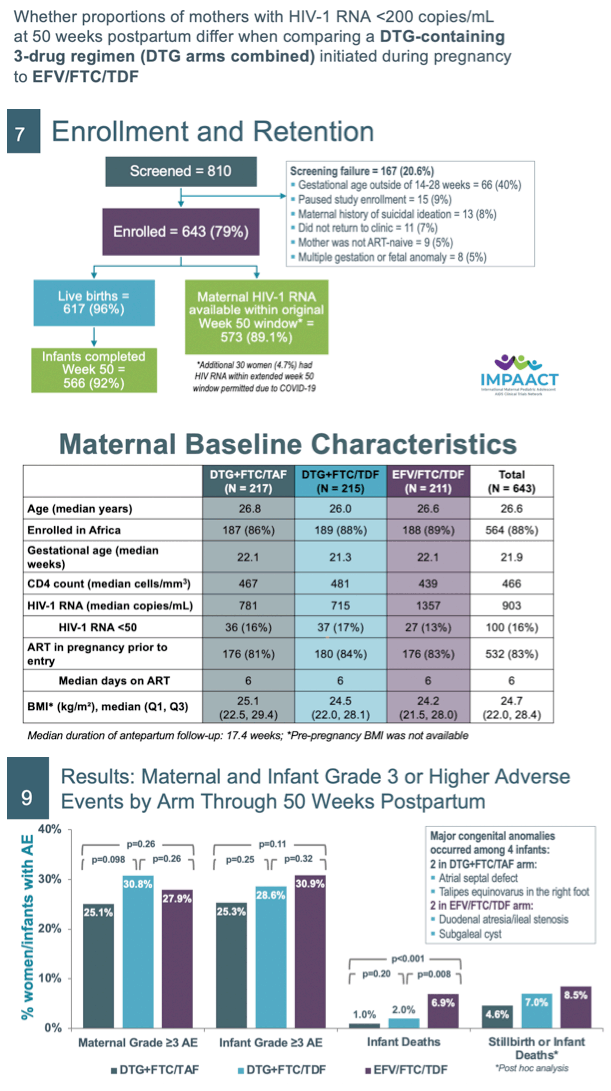
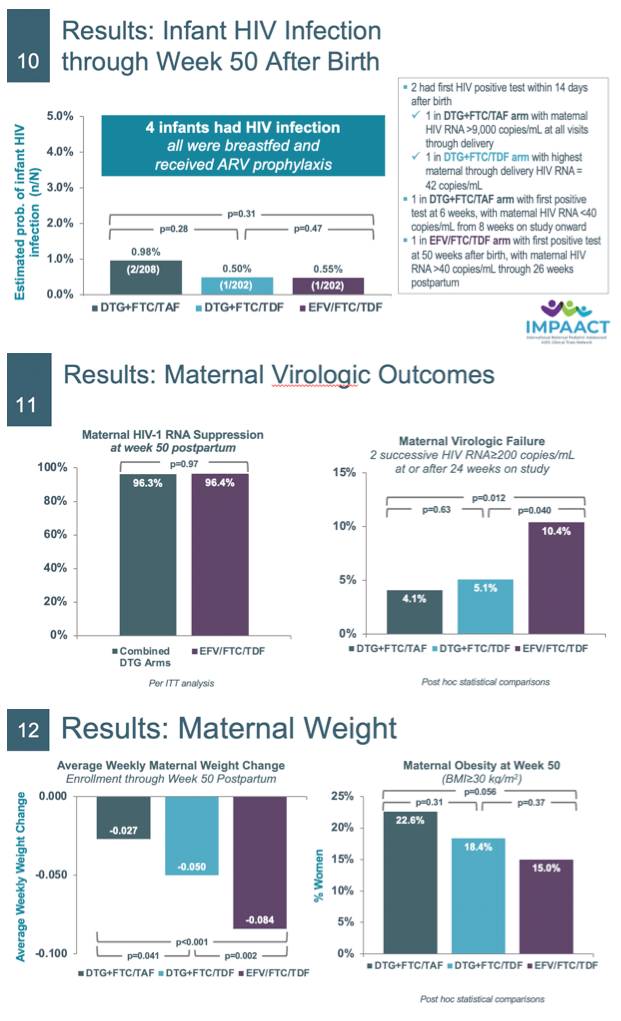
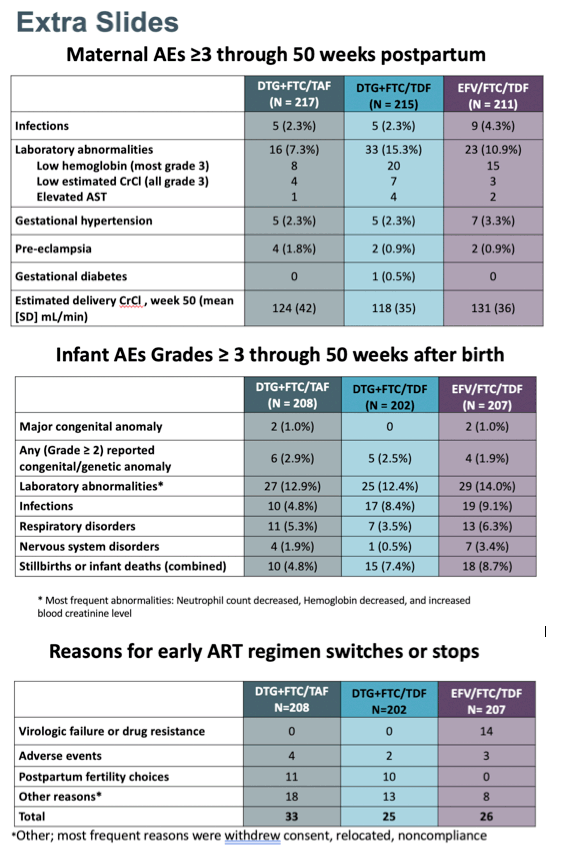
Results: We randomized 643 women: DTG+FTC/TAF (N=217), DTG+FTC/TDF (N=215), and EFV/FTC/TDF (N=211). Baseline medians: GA 21.9 weeks, HIV RNA 903 cp/mL, CD4 count 466 cells/uL. Six hundred and seven (94.4%) women and 566 (91.7%) of 617 liveborn infants completed the study; 479 (77.6%) infants breastfed (median 49.9 weeks). There were no apparent differences through week 50 PP between arms in the estimated probability of maternal grade≥3 AEs or infant grade≥3 AEs (Table 1). The average change in women's weight from entry through PP was -0.027 kg/week in DTG+FTC/TAF, -0.050 kg/week in DTG+FTC/TDF, and -0.084 kg/week in EFV/FTC/TDF arms. The estimated probability of infant death was higher in the EFV (6.9%) compared to DTG+FTC/ TAF (1.0%, p<0.001) and DTG+FTC/TDF (2.0%, p=0.008) arms. Either stillbirth (previously reported) or infant death (combined) occurred as follows: 10 in DTG+FTC/TAF, 15 in DTG+FTC/TDF, and 18 in EFV/FTC/TDF arms. Four infants were diagnosed with HIV: 2 in DTG+FTC/TAF, 1 in DTG+FTC/TDF, and 1 in EFV arm. At 50 weeks PP, proportions of women with HIV RNA <200cp/mL were similar in the combined DTG arms (96.3%) and EFV arm (96.4%). Regimen stops or switches were more frequent in the EFV arm due to virologic failure/drug resistance, and more frequent in the DTG arms due to PP fertility choices.
Conclusion: At week 50 PP, maternal and infant grade≥3 AEs from enrollment through week 50 PP were similar across arms; infant mortality was higher (though stillbirths somewhat less frequent) in those whose mothers were in the EFV/FTC/TDF arm. Maternal HIV RNA suppression was similarly high in the combined DTG vs the EFV arm, although more women stopped EFV due to virologic failure.


|
| |
|
 |
 |
|
|