 |
 |
 |
| |
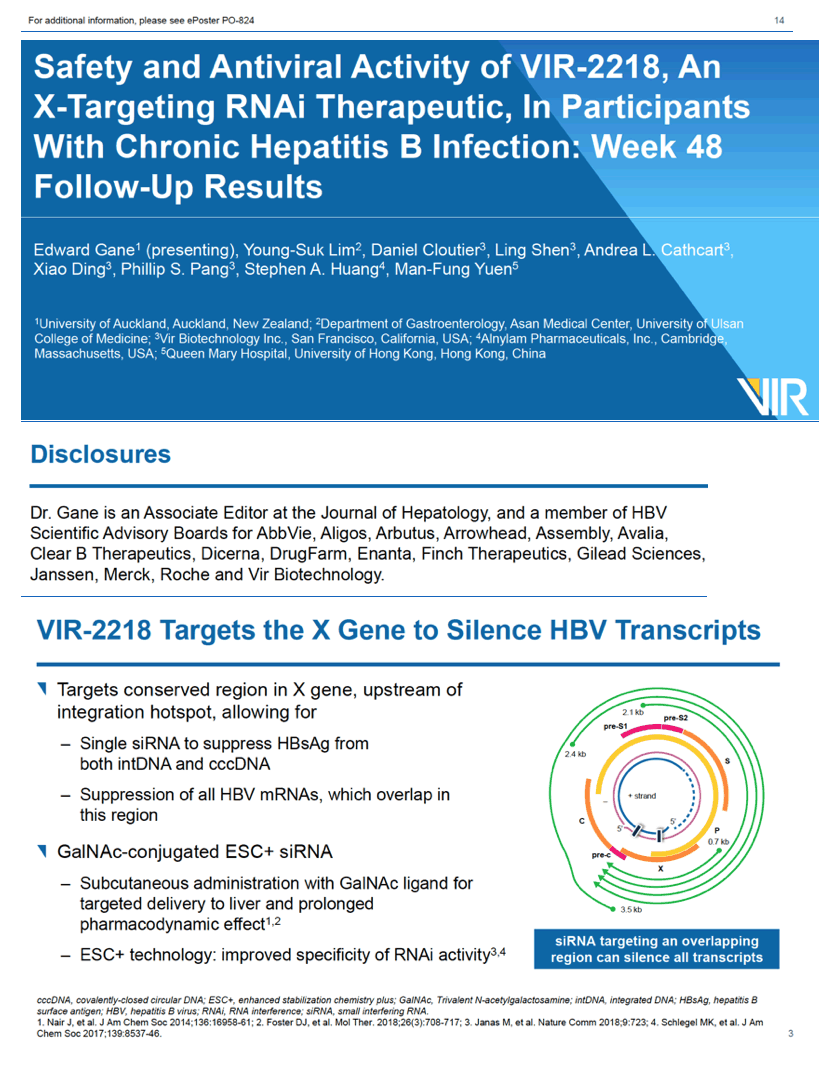
Safety and antiviral activity of VIR-2218, an X-targeting RNAi therapeutic, in participants with chronic hepatitis B infection: week 48 follow-up results
HBV Functional Cure Candidate, an siRNA, Passes Phase 2 Test of Activity
|
| |
| |
EASL International Liver Congress, June 23-26, 2021
Mark Mascolini
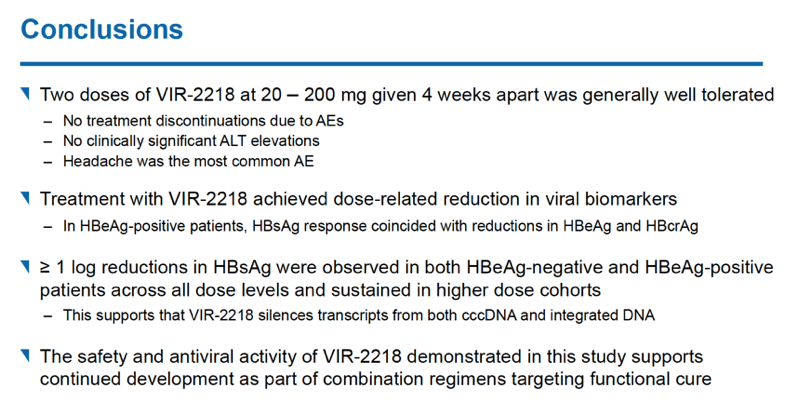
VIR-2218, a small interfering ribonucleic acid (siRNA) and a candidate for functional cure of chronic hepatitis B virus (HBV) infection, induced substantial drops in hepatitis B surface antigen (HBsAg) when given subcutaneously twice over 4 weeks [1]. None of 24 participants dropped out of the study because of adverse events.
This novel siRNA targets a conserved region of the HBV X gene, aiming to stifle production of all HBV proteins, including hepatitis B surface antigen (HBsAg), a marker of current HBV infection [2]. Vir Biotechnology, the developer of VIR-2218, believes that combining an agent like this with other anti-HBV therapies could allow many people with chronic HBV to attain functional cure, in other words, life-long control of HBV without life-long therapy [2]. VIR-2218 is designed to shut down all major HBV transcripts in all 10 HBV genotypes.
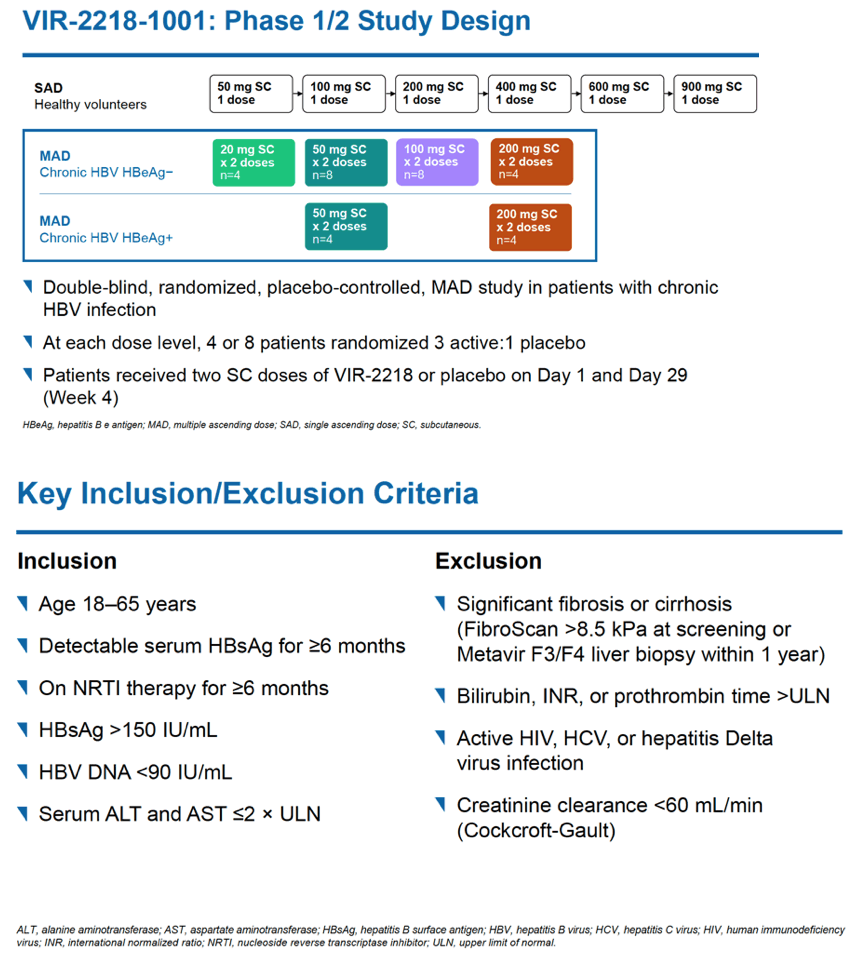
This phase 2 trial enrolled adults with virologically controlled chronic HBV infection and without significant fibrosis or cirrhosis or active HIV, HCV, or hepatitis Delta virus infection. Participants had to be 18 to 65 years old and have detectable HBsAg for 6 months or longer, current HBsAg above 150 IU/mL, HBV DNA below 90 IU/mL, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) at or below 2 times the upper limit of normal. Everyone had taken nucleos(t)ide therapy for at least 6 months.
Researchers randomized participants in a 3-to-1 ratio to get subcutaneous doses of VIR-2218 or placebo on day 1 and on day 29. Among people assigned to VIR-2218, HBeAg-negative participants received 20, 50, 100, or 200 mg of the siRNA, while HBeAg-positive people got 50 or 200 mg. (HBeAg circulates in blood when HBV is actively replicating.)
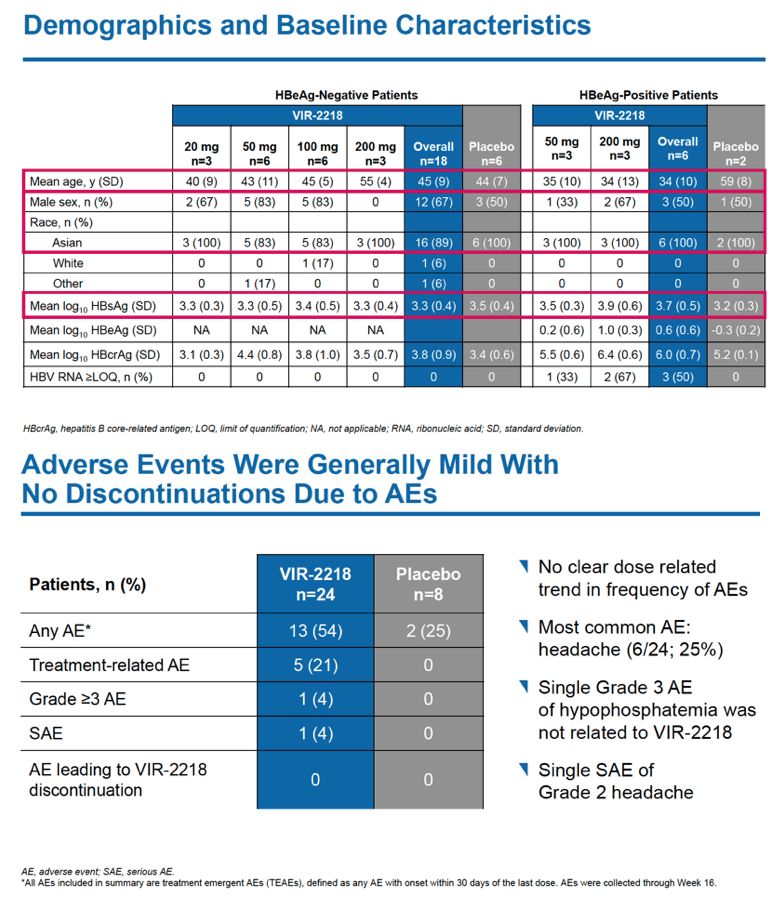
The trial enrolled 24 people, 18 HBeAg-negative and 6 HBeAg-positive. Most participants were male and Asian, findings reflecting demographics in HBV populations in countries involved in the study. Age averaged 45 in the 18 HBeAg-negative people and 34 in the 6 HBeAg-positive people.
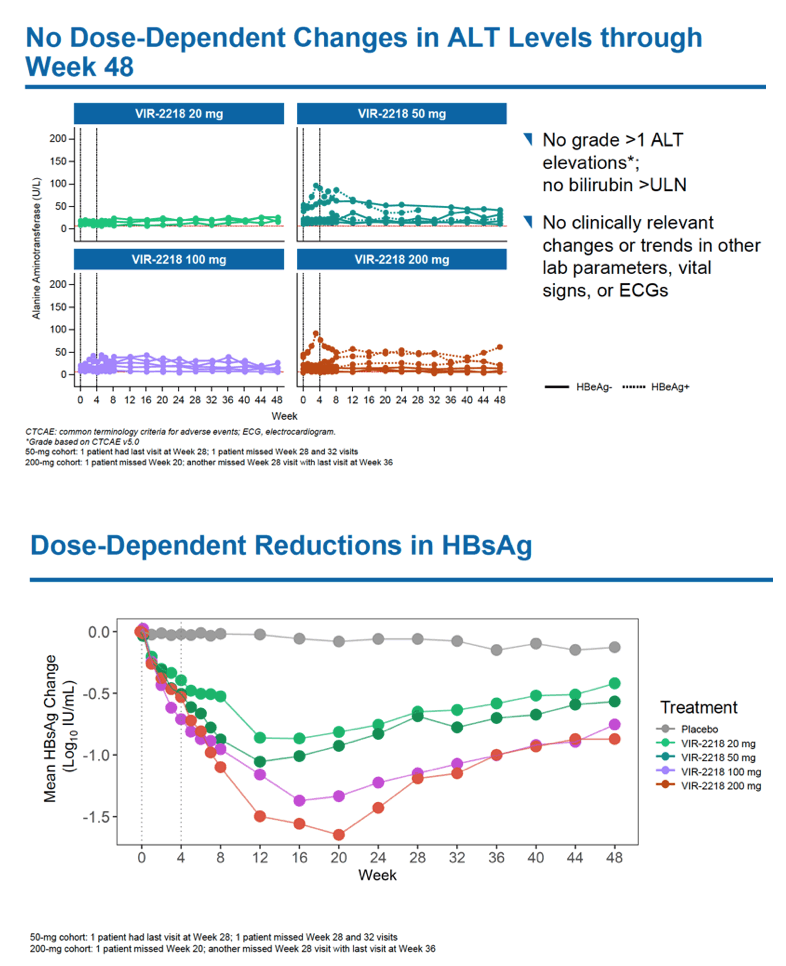
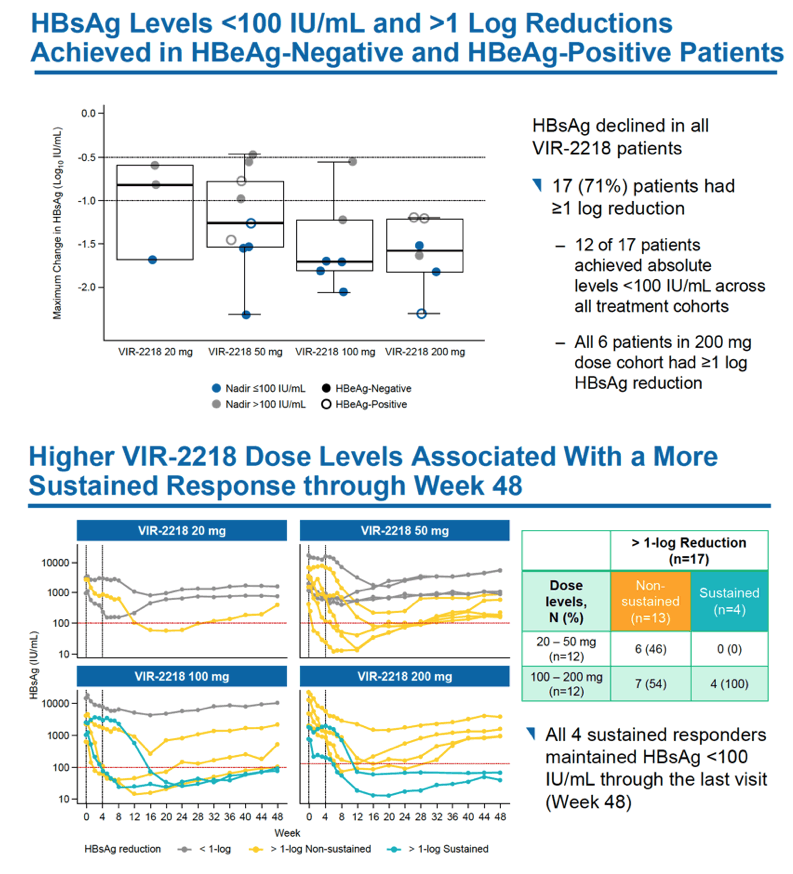
HBsAg levels fell over 48 weeks in a dose-dependent manner in people receiving VIR-2218 but did not change in the placebo group. Among HBeAg-negative participants, HBsAg load through 48 weeks dropped average maximums of 1.03 log10 IU/mL with 20 mg of VIR-2218, 1.23 log10 with 50 mg, 1.50 log10 with 100 mg, and 1.65 log10 with 200 mg. Among HBeAg-positive people average maximum drops in HBsAg through 48 weeks measured 1.16 log10 IU/ml with 50 mg of VIR-2218 and 1.57 log10 with 200 mg. Most participants randomized to VIR-2218 reached their maximum HBsAg decline by treatment week 16.
Seventeen of 24 participants receiving VIR-2218 (71%) had at least a 10-fold drop in HBsAg level, including all 6 people taking the 200-mg dose. Twelve people (50%) reached an HBsAg concentration below 100 IU/mL.
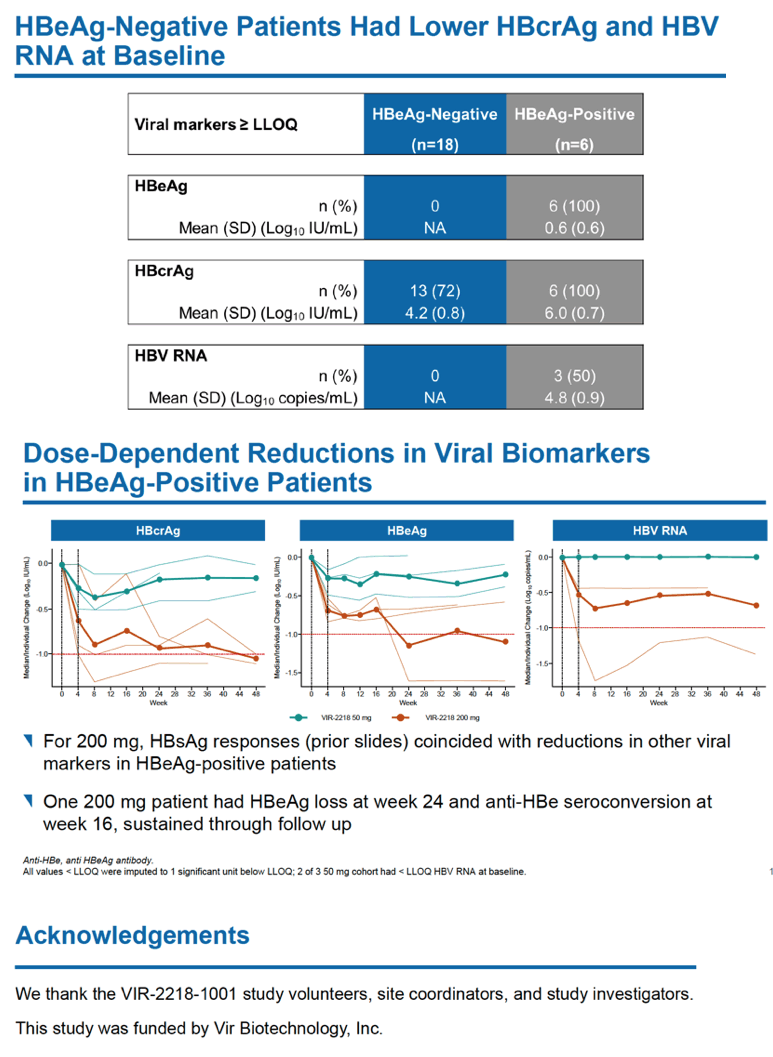
Because the trial recruited people with controlled HBV infection, levels of HBV DNA and HBV RNA were below measurable limits when this trial began, and treatment with VIR-2218 did not significantly change HBV DNA or HBV RNA status. One person receiving 200 mg of VIR-2218 had HBeAg loss at week 24 and anti-HBe seroconversion at week 16 and through follow-up.
No one dropped out of the 48-week trial because of an adverse event, and most treatment-emergent adverse events were mild. No study participant had clinically meaningful jumps in ALT or dose-dependent changes in ALT through week 48. Five people taking VIR-2218 (21%) had a treatment-related adverse event, 1 (4%) had a grade 3 adverse event (hypophosphatemia not related to VIR-2218), and 1 had a serious adverse event. The researchers saw no clear relationship between dose and adverse event frequency.
That HBsAg levels fell substantially in both HBeAg-negative and -positive people receiving VIR-2218 suggested to the researchers "that VIR-2218 may silence transcripts from both cccDNA and integrated DNA." (Formation and persistence of HBV covalently closed circular DNA (cccDNA) "is the root cause of HBV chronicity" [3]. Integrated DNA is HBV DNA integrated into the cellular genome [4].)
VIR-2218 will be combined with VIR-3434, a human antibody against HBsAg, in the MARCH trial.
References
1. Gane E, Lim YS, Cloutier D, et al. Safety and antiviral activity of VIR-2218, an X-targeting RNAi therapeutic, in participants with chronic hepatitis B infection: week 48 follow-up results. EASL International Liver Congress, June 23-26, 2021. Abstract OS-44.
2. VIR. Pipeline. VIR-2218. https://www.vir.bio/pipeline/
3. Wei L, Ploss A. Hepatitis B virus cccDNA is formed through distinct repair processes of each strand. Nature Communications. 2021;12:article number 1591.
4. Zhao K, Liu A, Xia Y. Insights into hepatitis B virus DNA integration-55 years after virus discovery. The Innovation. 2020;1(2):10034. https://doi.org/10.1016/j.xinn.2020.100034
|
| |
|
 |
 |
|
|