 |
 |
 |
| |
Summary from EASL 2021 for Hepatitis
B, C, D and fatty liver disease.
Liver disease in 2021: advances and remaining challenges.
|
| |
| |
Jurgen K. Rockstroh M.D., Professor of Medicine
University of Bonn, Germany
Prof. Dr. J.K. Rockstroh
Department of Medicine I
University Hospital Bonn
Venusberg-Campus 1
53127 Bonn
Germany
Introduction
The International Liver Congress™ (ILC) of the European Association for the Study of the Liver (EASL) for the second time took place virtually from June 23rd-26th 2021. Over 6.000 delegates online documented the persistent interest in chronic liver diseases, even though the opportunity to meet face-to-face with colleagues from all over the world was deeply missed. Overall, 1,500 abstracts were accepted, and more than 103 countries were represented at this truly international meeting. There was an amazing amount of data around new HBV and HDV treatments, which promise a growing treatment armentarium for these viral infections. Although, no new HCV drugs are entering the HCV treatment arena anymore, there was a considerable amount of new data discussing the global progress in achieving HCV elimination as well as the course of liver disease after achieving successful HCV cure after all oral DAA therapy. Numerous presentations were around new drugs in the pipeline for treatment of NAFLD and NASH. In addition, the newly revised EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 were released for the first time and the corresponding publication is now downloadable from the EASL website (https://easl.eu/wp-content/uploads/2018/10/Non-invasive-English-report.pdf) (1). Finally, the impact of the ongoing COVID-19 pandemic on the outcome of patients with underlying liver disease but also impact on HCV testing services and treatment plans were presented. Also need for prioritized vaccination in patients with liver diseases as well as vaccine response rates in some vulnerable patient populations with underlying liver disease were discussed. The conference platform was easy to navigate and all poster presentations remain downloadable. Many oral sessions are still viewable as on demand presentations and there are best of ILC 2021 slide decks available online. Therefore, compliments to the conference organizers for making access to new research in chronic liver disease possible in difficult times.
Management of chronic hepatitis B: anything new?
Differential relapse patterns have been observed following discontinuation of tenofovir disoproxil fumarate (TDF) or entecavir (ETV) in chronic hepatitis B (CHB) patients (2). Whether the type of nucleos(t)ide analogue (NA) (ETV or TDF) affects rates of off-therapy hepatitis B surface antigen (HBsAg) loss however, remains unclear. At this year ILC meeting data from the RETRACT-B study was presented which studied rates of HBsAg loss, virological and clinical relapse, and retreatment following discontinuation of ETV or TDF therapy (3). Individual patient data meta-analysis of CHB patients who stopped either TDF or ETV therapy between 2001 - 2020 from 12 participating centers across North America, Europe, and Asia was conducted. All included patients were hepatitis B e antigen (HBeAg) negative with undetectable serum HBV-DNA at treatment discontinuation. Patients were analyzed for the following outcomes: HBsAg loss, virological relapse (VR) defined as HBV DNA ≥2000 IU/mL or clinical relapse (CR) defined as HBV DNA ≥2000 IU/mL and ALT ≥2 X ULN. Unweighted analysis assessed rates of outcomes using univariable Cox regression, and weighted analysis estimated inverse probability of treatment weights (IPTW) with propensity scores used to balance treatment groups (groups were balanced on age, sex, race, NA therapy duration, treatment history, HBeAg status at start of therapy, cirrhosis, ALT and HBsAg at end of therapy). Overall, during a median off-treatment follow-up of 18 months HBsAg loss was observed in 96 (6.8%) patients. Virological and clinical relapse occurred in 1097 (78%) and 598 (43%) patients, respectively. 667 (48%) patients were retreated. Unweighted and weighted rates of outcomes (HBsAg loss, VR, CR, and retreatment) comparing TDF to ETV group are shown in figure 1.
Figure 1: Unweighted and weighted rates of outcomes from the RETRACT-B study.
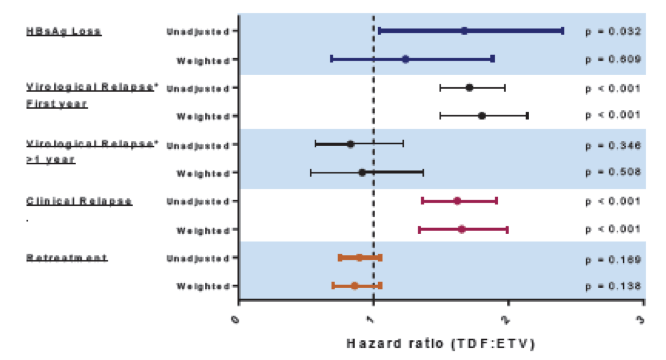
Type of treatment (ETV or TDF) did not significantly impact HBsAg loss rate after statistical adjustment. Virological and clinical relapse however, were observed earlier and more frequently in patients treated with TDF versus ETV, with all measured variables balanced. Virological relapse rates in the two groups became comparable after first year off-therapy and retreatment rates did not differ between the treatment groups. Overall, HBsAg loss still remains a relatively rare event after discontinuing nucleos(t)ide therapy and virological relapse clearly occurs in the majority of patients. Therefore, NUC-specific post-therapy monitoring is necessary. As relapse rates appear to become similar after 12 months between the two HBV therapies, the clinical implication of earlier rebound with TDF remains uncertain.
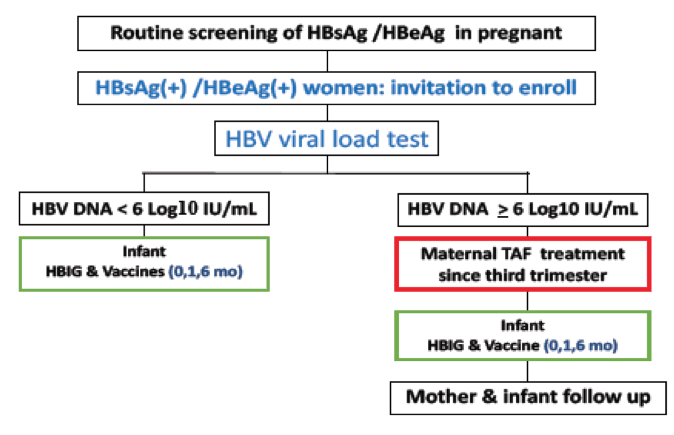
The major route of chronic hepatitis B infection in the immunization era is mother-to-infant transmission (MTIT) during perinatal period. HBV transmission is most likely from mothers with hepatitis B surface antigen (HBsAg)/ hepatitis B e antigen (HBeAg) positivity and high maternal HBV viral load. Short-term antiviral therapy for pregnant women with chronic hepatitis B virus infection and high viral load has been recommended to prevent maternal transmission. Antiviral therapy using tenofovir disoproxil fumarate (TDF) or telbuvudine since third trimester of pregnancy has been shown to reduce HBV infection in children born to high viral load mothers. Data of using tenofovir alafenamide fumarate (TAF) treatment for preventing mother-to-infant transmission however, is limited. At this year ILC meeting data from a clinical trial using TAF for preventing mother-to-infant transmission of HBV, in comparison with mothers using TDF treatment was presented (4). Of note these were only results from an interim analysis as the study is still ongoing. So far, this prospective, multi-center clinical trial recruited 57 HBV-infected pregnant women between 20-45 years of age, who were seropositive for HBsAg and HBeAg and had an HBV viral load above 1,000,000 IU/mL. Enrolled subjects received TAF 25 mg daily (TAF group) since third trimester until 2 weeks postpartum in 2019-2021. Maternal and children's outcomes were compared with 53 pregnant women who received TDF 300 mg daily (TDF group) during 2016- 2018. All infants received HBIG and HBV vaccine at birth, and at 6 and 12 months. The study flow is summarized in figure 2.
Figure 2: Flow chart of maternal screening, TAF treatment, and infant follow-up
A total of 57 pregnant woman received TAF treatment. The HBV DNA levels decreased from 7.87 ± 0.57 Log10IU/mL at baseline, to 3.96 ± 1.10 Log10IU/mL at delivery in the TAF group; and from 8.30 ± 0.36 Log10IU/mL to 4.47 ± 0.86 Log10IU/mL in the TDF group. The mean reduction of HBV DNA levels was comparable between the TAF group (3.90 ± 0.90 Log10IU/mL) and TDF group (3.83 ± 0.83 Log10IU/mL, p= 0.67). 2. The percentage of the mothers to achieve HBV DNA level below 6.0 Log10IU/mL at the time of delivery were 94.7% (54/57) in the TAF group, versus 96.2% (51/53) in the TDF group (p=1.00). Safety of TAF and TDF overall was very good. Of the mother in the TAF group, one experienced nausea and one developed dry eyes. All symptoms subsided spontaneously. In the TDF group, two mothers experienced nausea, one with skin itching and one with mild diarrhea. Of the 62 newborns delivered in the TAF group, the average gestation age was 38.3 ± 1.8 weeks; and 38.7 ± 1.3 weeks of the 55 newborns in the TDF group (p= 0.17). The mean body weight was 2958 ± 520 gram in the TAF group and 3052 ± 431 g (p= 0.30) in the TDF group. Of the 42 infants in the TAF group followed up to 6 months, one was HBsAg positive (1/42), versus one (1/55, p= 1.00) in the TDF group. Of the 32 infants in the TAF group followed up to 12 months, none were HBsAg positive (0/32), versus one (1/55, p= 1.00) in the TDF group. The course of viral load reduction in pregnant women using TAF or TDF is shown in figure 3.
Figure 3: HBV Viral load reduction in pregnant women using TAF or TDF
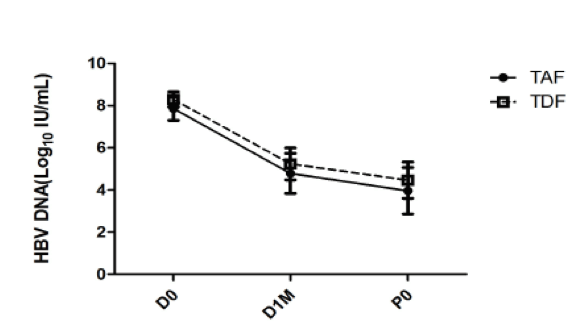
(D0: baseline; D1M: 1 month after TAF or TDF treatment; P0: at delivery, approximate 2 months after TAF or TDF treatment)
In summary, the effects of TAF treatment for highly viremic HBV-infected pregnant women were comparable with TDF treatment in terms of maternal HBV DNA reduction and in preventing mother-to-infant transmission.
Additional data regarding the administration of tenofovir alafenamide (TAF) during pregnancy for the prevention of MTIT of HBV were presented at 2021 ILC meeting from China (5). In this ongoing, multi-center, prospective study, chronic HBV-infected pregnant women aged from 20 to 35 years old, positive for Hepatitis B e-antigen and with HBV DNA level at least 1X106 IU/mL, received oral TAF (25 mg/day) from gestational weeks 24-28 until postpartum week 4. All infants received HBV immunoprophylaxis. The participants, including mothers and infants, were followed up until postpartum week 28. The primary measurement was MTIT rate. The key secondary measurement was the safety of TAF for mothers and infants. A total of 89 of 91 enrolled pregnant women received TAF treatment. At present, all mothers already delivered. TAF therapy was initiated at a mean gestational age of 25.04(±0.96) weeks with the mean treatment duration of 14.3(±1.2) weeks before delivery. 92.1% (82/89) mothers discontinued the TAF treatment, the median time was 5.9 weeks. Safety was good with no serious adverse events recorded. At delivery, 82.0% mothers achieved HBV DNA <2X105 IU/ml, and 21.4% achieved HBV DNA <500 IU/ml. Overall, 91 infants (two sets of twins) were born. No infant (0/91) had congenital defects. So far, 79 and 48 infants were followed up to postpartum week 28 and 48, respectively. The HBsAg positive rates were 0% at 28 weeks in 79 infants and 48 weeks in 48 infants, including the 3 infants with HBsAg positive, respectively at birth. No growth retardation was detected at any time.
In conclusion, TAF therapy initiated during the second trimester for HBV-infected pregnant women with positive HBeAg and high HBV DNA level was effective in preventing MTIT, and there were no safety concerns for mothers and infants with 28 weeks of follow up after delivery. The long-term benefits of using TAF in MTIT, when compared with TDF, particularly on bone and renal safety, remain to be explored.
Hepatitis B immunoglobulin (HBIG) is a purified solution of human immunoglobulin that can be administered to the mother, newborn, or both. Indeed, HBIG has been recommended by guidelines for use in infants born to HBsAg positive mothers especially if they are HBeAg positive or with high HBV DNA. Indeed, in the first trial on MTIT discussed above all infants received HBIG administration as well as HBV vaccination as part of the study protocol. HBIG, however is costly and not available everywhere. In Cambodia intervention to prevent mother-to-child transmission (MTCT) of hepatitis B virus is limited to childhood vaccine, for which national coverage is 88.7% (45% for timely birth-dose). Most interestingly, at the ILC meeting data was presented from a trial in Combodia which evaluated an HBIg-free strategy for preventing mother-to-child-transmission using a TDF prophylaxis (6). The decision about use of TDF prophylaxis was based on the results of HBsAg/HBeAg rapid diagnosis tests and ALT levels for those eligible, from 24 weeks of amenorrhea and "test and treat" strategy for those seen later during pregnancy. Following a protocol amendment from 2018 onwards, HBeAg-negative and ALT≥40 IU/L received TDF from 24 weeks of amenorrhea to 6 weeks postpartum. HBIg was not recommended but could be done if accessible. All infants received early HBV vaccination at birth. The study flow of the TA-PROHM study is shown in figure 4.
Figure 4: Study flow in the TA-PROHM study
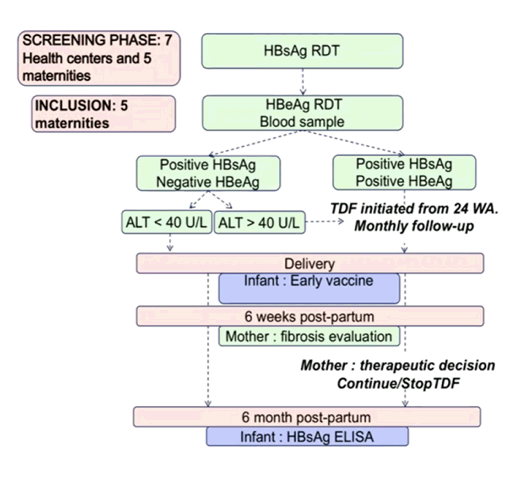
Primary endpoint was the proportion of active HBV infection in infants at 6 months of life estimated by plasmatic HBsAg positivity conformed by HBV-DNA determination. From 2017 to 2019, 21,251 women were screened for HBsAg and 1,339 (6.3%) were positive. 1,194 women were enrolled of whom 338 (28.3%) were eligible for TDF prophylaxis. At enrollment, median age was 29 years and median gestational age 23 weeks. The median HBV
DNA level was 7.9 log10 IU/ml for TDF-eligible women and 2.5 log10 IU/ml for non TDF-eligible women. The proportion of eligible women starting TDF was 94.1%. At birth, 86.5% of infants received the first dose of vaccine ≤2 hours of life, 95.5% ≤24 hours of life and 15.4% received HBIg. Overall, 4/317 infants (1.26%, 95% CI 0.34-3.20) were infected in the TDF group and 7/711 infants (0.98%, 95% CI 0.4-2.02) in the non TDF group. For infants not receiving HBIg, 4/271 (1.48%, 95% CI 0.40-3.74) were infected in the TDF group and 6/565 (1.06%, 95% CI 0.39-2.30) in the non TDF group. Transmission rate for women treated by TDF for ≥1 month (N = 259) was 0% (95% CI 0-1.41). the results for the primary endpoint are summarized in table 1.
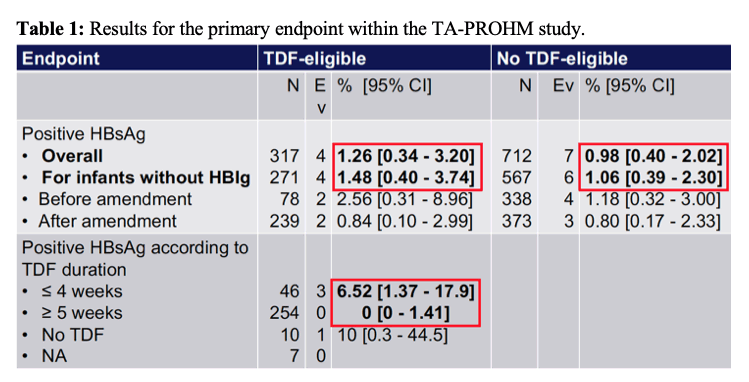
In conclusion, these results demonstrate that an HBIg-free strategy with maternal use of TDF and early vaccination at birth could be effective to reduce HBV MTCT, specifically if TDF is administered for ≥1 month prior delivery. For women with low-level viremia, an early vaccination without HBIg could be enough to reduce HBV MTCT. These study findings could have a major health impact by allowing decentralization of this strategy at the district level, where most pregnant women in Cambodia are followed. Clearly the outlined rapid diagnostic based treatment decision algorithm with TDF appears to be feasible and associated with low costs.
HBV therapy with nucleos(t)ide analogue (NA) therapy is well-known to decrease the risk for development of hepatocellular carcinoma. Whether HBV therapy with nucleos(t)ide analoques also has an impact on the incidence of extrahepatic malignancies is still unclear. In the oral abstract session data was presented from an 18-month landmark study using the nationwide claim data from the National Health Insurance Service of Korea, which evaluated the effect of HBV nucleos(t)ide analogue therapy on the development of extrahepatic malignancies in HBV patients (7). 90.944 Patients who were diagnosed with chronic hepatitis B (CHB) between 2012-2014 and matched-controls from general population (n = 685, 436) (matched by age, sex, socioeconomic status, and areas of habitat) were included into the study. CHB patients were further classified into NA-treated (n = 6,539) or NA-untreated CHB groups (n = 84,405). Washout period was established two years prior to the cohort entry date. The inverse probability treatment weighting analysis was applied for balancing study groups. The primary outcome of the study was the development of overall extrahepatic malignancy. The development of liver cancers (HCC and intrahepatic cholangiocarcinoma) and death were considered as competing events. The median follow-up period (IQR) was 47.4 months (IQR=38.1-57.1 months). During the follow-up period 30.413 patients (3.8%) developed an extrahepatic malignancy. The NA-untreated CHB group had significantly higher risk of overall extrahepatic malignancy than both the NA-treated CHB group (adjusted sub-hazard ratio [aSHR] = 1.27, 95% confidence interval [CI] = 1.11-1.45, p = 0.003) and the control group (aSHR = 1.22, 95% CI =1.18-1.26, p <0.001), while there was no difference between the NA-treated CHB group vs. the control group (NA-treated vs. control, aSHR = 0.96, 95% CI = 0.84-1.09, p = 0.55). Sensitivity analysis using different landmarks confirmed the observed differences in incidence rates for extrahepatic malignancies between the different groups. The incidence of extrahepatic malignancies by group is depicted in figure 5.
Figure 5: Cumulative incidence of extrahepatic malignancy (18-months
landmark) before inverse probability treatment weighting.
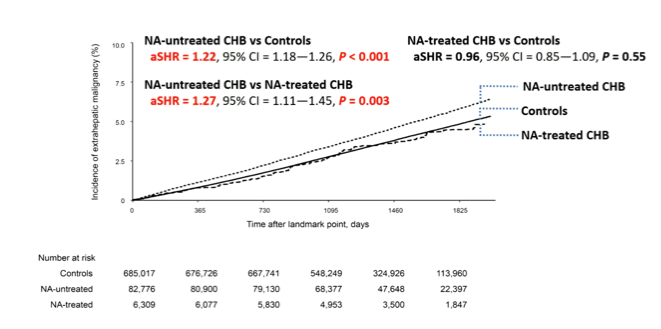
This very large study clearly demonstrates that patients with chronic hepatitis B without HBV nucleos(t)ide treatment have a significantly higher risk for developing extrahepatic malignancies than the general population. HBV patients under nucleos(t)ide HBV therapy however, have their risk for extrahepatic malignancies attenuated and which is comparable to the risk for extrahepatic malignancies in the general population.
In patients with chronic hepatitis B, TAF appears to be as effective as TDF, with lower bone and renal toxicity. TAF however, has the potential advantages that dose adjustment is not required in patients with renal impairment, and monitoring can be less intense because of the better safety profile. The phase 2 study (NCT03180619) evaluated efficacy and safety 96 weeks after switching to TAF in virally suppressed chronic hepatitis B patients with moderate-severe renal insufficiency (RI) or end-stage renal disease (ESRD) maintained on hemodialysis (8). This study enrolled virally suppressed chronic hepatitis B patients (HBV DNA < LLOQ X ≥ 6 months and <20 IU/ml at screening) with moderate or severe RI or with ESRD on HD at screening while receiving TDF and/or other oral antivirals for ≥ 48 weeks. All study participants were then switched to TAF 25 mg QD for 96 weeks. Safety assessments including adverse events (AEs) and changes in bone (hip and spine BMD) and renal (creatinine clearance by Cockcroft Gault [eGFRCG], serum phosphorus, and serum creatinine -except in ESRD patients) parameters, viral suppression, and serological and biochemical responses were serially assessed. Overall 93 subjects were enrolled, 78 (cohort 1) with moderate-severe renal insufficiency and 15 (cohort 2) who were already on hemodialysis. Table 2 summarizes the main efficacy results for both cohorts (M=F missing equals failure and M=E missing equals exclusion approach).
Table 2: Efficacy results at week 96 by study group.
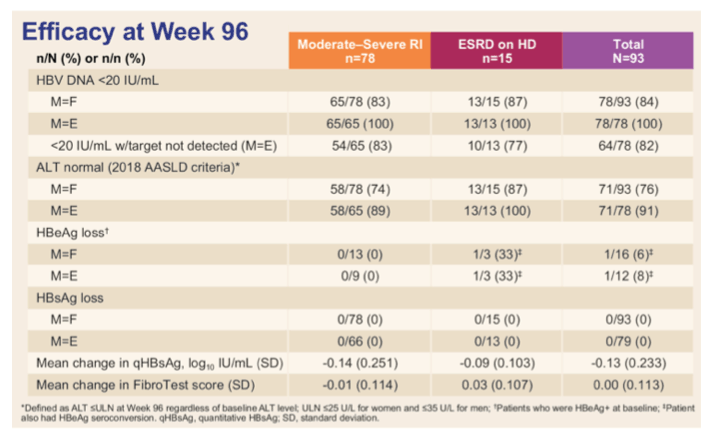
Viral suppression was well maintained up to 96 weeks. ALT levels also remained normal in nearly all patients while serological responses were low. TAF overall, was safe and well tolerated; the incidences of grade 3 and 4, and serious adverse events were reflective of patients with renal disease and its accompanying comorbidities. Renal function (eGFRCG) remained stable in patients with moderate-severe renal insufficiency with sustained decrease in markers of tubular function (see figure 6).
Figure 6: Renal parameter results over 96 weeks in cohort 1
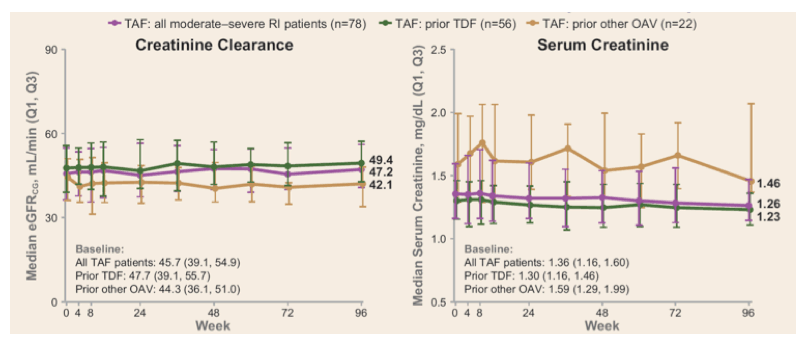
In summary, renally-impaired CHB patients, including ESRD patients on HD, who were switched to TAF from TDF and/or other OAVs maintained high rates of viral suppression, and bone and renal parameters remained stable or slightly improved after 2 years of treatment.
Hepatitis B therapy: what are the new drug developments?
At this year ILC meeting there were plenty of presentations on various new drugs and immunotherapies for HBV therapy and as part of a potential future HBV cure strategy. Not all studies can be presented here but a selection of very interesting new treatment approaches will be discussed below.
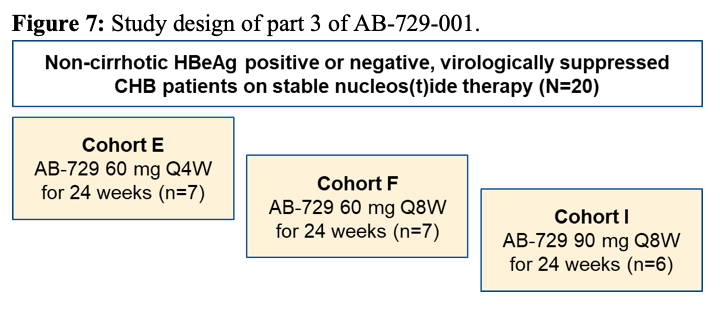
Current hepatitis B drug treatments can suppress viral replication, slow the progression of liver fibrosis, and reduce infectivity, but can rarely clear the viral covalently closed circular DNA (cccDNA) that is responsible for HBV persistence. Alternative therapeutic strategies, including those based on viral gene silencing by harnessing the RNA interference (RNAi) pathway, effectively suppress HBV replication and thus hold promise. RNAi-based silencing of certain viral genes may even lead to disabling of cccDNA during chronic infection. Indeed, many companies have moved into the HBV cure space and are looking at different interventions to tackle the HBV replication cycle. Within the late-breaker session new data on AB-729 an N-Acetylgalactosamine (GalNAc)-conjugated single trigger RNA interference therapeutic that blocks all HBV RNA transcripts, including HBx, resulting in suppression of viral replication and all viral antigens, was discussed (9). AB-729 is currently in clinical development for the treatment of chronic hepatitis B (CHB). At this year ILC meeting the preliminary safety and pharmacodynamic (PD) results with repeat dose administration of AB-729 subcutaneously in chronic hepatitis B subjects was presented. In AB-729-001 Part 3, 20 non-cirrhotic, HBeAg positive or negative, virologically-suppressed CHB subjects on stable nucleos(t)ide therapy received AB-729 60mg every 4 weeks (Q4W, Cohort E, N=7), 60mg every 8 weeks (Q8W, Cohort F, N=7), or 90mg Q8W (Cohort I, N=6) through Week 24. Eligible subjects ( >0.5 log10 HBsAg reduction at Week 20) had the option to continue AB-729 through Week 48: Cohort E switched to AB-729 60mg every 12 weeks (Q12W) while Cohorts F and I maintained their initial AB-729 regimen. The corresponding study design for part 3 of this study is summarized in figure 7.
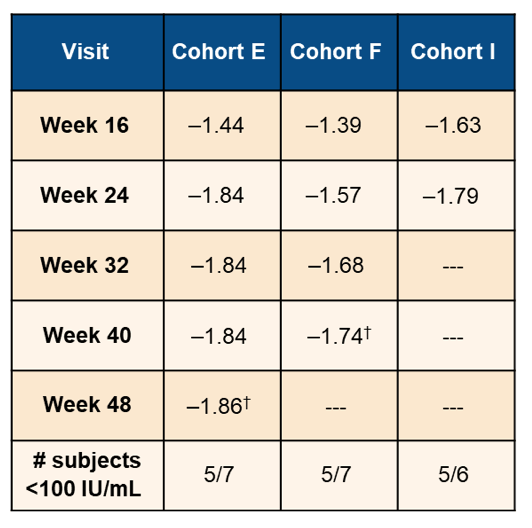
There was no difference in mean HBsAg response between AB-729 doses and dosing intervals to date (p≥0.2). A plateau occurred around Week 20 with no evidence of HBsAg rebound so far. Overall, to date, 15/20 subjects attained HBsAg <100 IU/mL. Table 3 highlights the mean Δlog10 HBsAg level changes from the study.
Table 3: Mean Δlog10 HBsAg level changes.
With regard to safety outcomes no deaths, SAEs, or Grade 3/4 treatment emergent adverse events (TEAE) or laboratory abnormalities were noted beyond a single transient Grade 3 creatine kinase elevation. Reassuringly, most TEAEs were Grade 1. 2 patients with diagnosed COVID-19 disease developed Grade 2 adverse events. 1 with accompanying fever. The most common TEAEs were injection-site AEs which on average were Grade 1, and none appeared to be dose- or interval-dependent. No ALT elevations were considered AEs by the investigators, and no bilirubin or liver synthetic function changes were observed.
In summary, AB-729 repeat dosing is generally safe and well tolerated. Robust mean declines in HBsAg were sustained with repeat dosing of AB-729, with most subjects reaching HBsAg<100 IU/mL and no meaningful differences observed to date between dose and/or dosing intervals. Development of AB-729 continues with assessment of longer dosing intervals (Q12W) as well as in combination with other agents.
Another interesting presentation on RNAi-based antiviral drugs in the oral abstract session was from a Phase 2 trial of VIR-2218 in participants with chronic hepatitis B (10). VIR-2218 is an investigational GalNAc-conjugated siRNA being developed for functional cure of CHB. At this year ILC meeting final safety and antiviral activity data were presented for different subcutaneous doses of VIR-2218 administered at day 1 and 29 with subsequent follow-up for 32 weeks. The study design is shown in figure 8.
Figure 8: Study design
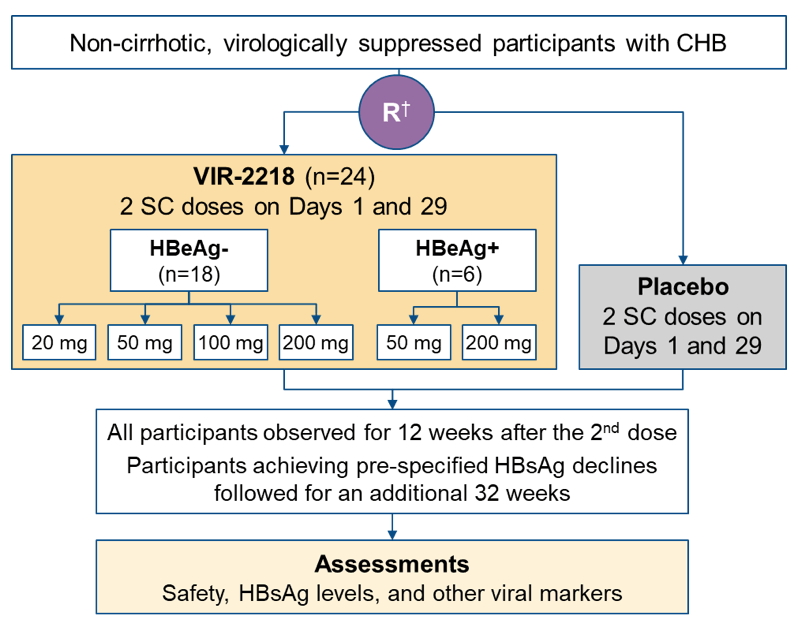
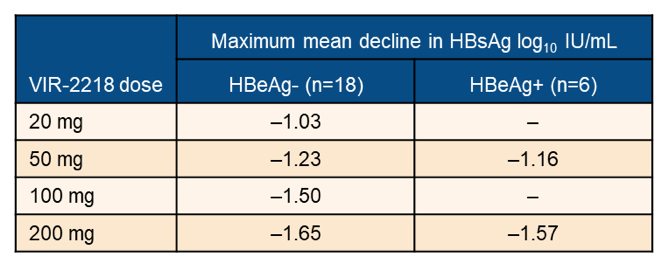
Non-cirrhotic, virologically suppressed participants received 2 subcutaneous doses of VIR-2218 or placebo on Day 1 and Day 29 (Week 4). HBeAg- participants received 20, 50, 100 or 200 mg, and HBeAg+ participants received 50 or 200 mg. Cohorts included 4 or 8 participants randomized 3:1 to VIR-2218 or placebo. Assessments included safety, HBsAg levels and other viral markers, with 12 weeks of follow-up after the 2nd dose for all participants and an additional 32-weeks follow-up for participants achieving pre-specified HBsAg declines. Twenty-four chronic hepatitis B participants received VIR-2218 (18 HBeAg-; 6 HBeAg+). The mean decline in HBsAg levels are shown in table 4.
Table 4: Results: Maximum mean decline in HBsAg log10 IU/ml by treatment dose and HBeAg status.
Most participants had levels of HBV DNA and HBV RNA < LOQ at baseline, and significant changes were not detected. The maximum HBsAg decline was observed mostly by week 16. Declines in qHBeAg and HBcrAg were observed in HBeAg+ subjects receiving 200 mg VIR-2218. No participants discontinued due to an adverse event (AE), and the majority of treatment emergent AEs were mild in severity. Moreover, no clinically significant ALT elevations were observed.
In conclusion, two doses of VIR-2218 at 20-200 mg given 4 weeks apart were well tolerated in CHB participants. Substantial reductions in HBsAg were observed in both HBeAg- and HBeAg+ participants across all dose levels, suggesting that VIR-2218 may silence transcripts from both cccDNA and integrated DNA. Longer follow-up will be needed to better define the impact and clinical utility of this treatment approach.
Current oral antiviral hepatitis b therapy leads to continuous suppression of viral replication with undetectable hepatitis B virus DNA, but has minimal effect on hepatitis b surface antigen levels. GSK3228836 (ISIS 505358) is a 2`-O-methoxyl modified antisense oligonucleotide targeting all HBV RNAs including pregenomic RNA, designed to induce cleavage of HBV RNA visa RNase H1. Previous phase 2 studies have demonstrated rapid reductions in HBsAg and HBV-DNA levels during 4 weeks of treatment (11). At this year ILC results from a post-hoc analysis were presented which investigated the correlation of systemic biomarkers with antiviral responses of GSK836 (12). Within a day of GSK836 treatment, a dose dependent change of plasma proteins related to tissue remodeling and immune responses such as collagen IV and IFN-gamma was observed. Of the 17 patients receiving 300 mg GSK836, 7 patients showed >2 log decline in HBsAg at day 29 and also demonstrated ALT elevation peaking around week 5. During the time-course of HBsAg decline or elevation of ALT, inductions of various soluble immune mediators such as MCSF, MIP1b, IgM, IP10, and MIG were observed, suggestive of the activation of innate and adaptive T and B cell responses. In conclusion, multiple immunological responses were triggered by GSK836 treatment and potential biomarkers associated with HBsAg response and ALT elevation were identified in responders. The IFN-gamma pathway appears to be central to mediating immunological responses during HBsAg decline leading to ALT elevation in responders.
Antiviral Therapy for delta hepatitis: what is new?
HDV is the most severe form of chronic viral hepatitis with a 2-3-fold increased risk of mortality compared to HBV monoinfection (13). Unfortunately, treatment of HDV has always been very limited. More recently, bulevirtide (BLV) as a first-in-class entry inhibitor has been conditionally approved in Europe for treatment of chronic HDV. Of note bulevirtide is administered subcutaneously qd. Bulevirtide is a linear 47-amino acid chemically synthesized lipopeptide that specifically binds to sodium taurocholate cotransporting polypeptide (NTCP) at the basolateral membrane of hepatocytes; NTCP is used by HBV and HDV to enter hepatocytes. So far BLV has been associated with a favorable safety and tolerability profile. At this year ILC the interim week 24 analysis of safety and efficacy of BLV administered subcutaneously at a dose of 2 or 10 mg qd in combination with pegylated interferon alfa-2a (PEG-IFNα) qwk relative to BLV 10 mg monotherapy was presented (14). The study design is shown in figure 9:
Figure 9: MYR204 Study Design
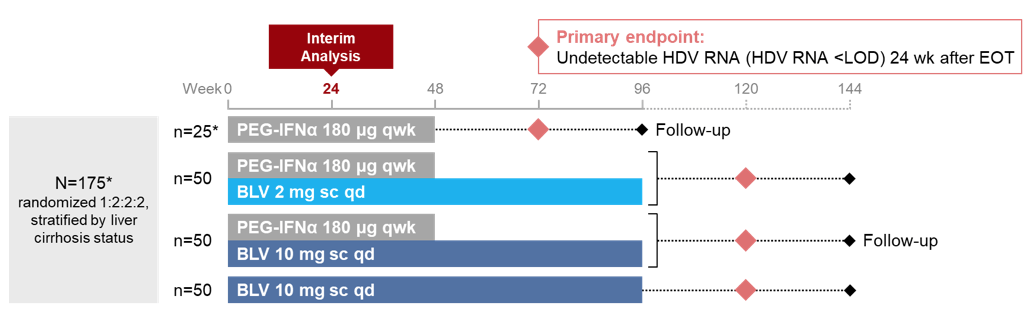
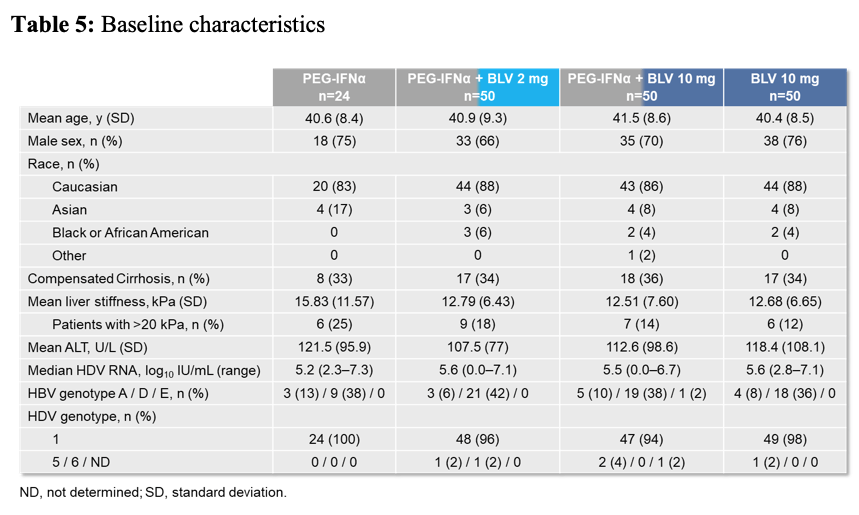
Overall, 175 HDV patients were included into the study. The main baseline patient characteristics are shown below in table 5.
Table 5: Baseline characteristics
Of note, one third of patients at baseline already had cirrhosis. In the first 24 weeks of treatment, 998 AEs were reported by 151 patients (86.8%), being mostly mild (536) or moderate (296). 181 of 998 AEs were judged as possibly related to BLV and 724 AEs to pIFN. Overall, 7 SAEs (judged as not related to BLV) were reported, one of which (anaplastic astrocytoma, judged as non-related to BLV nor pIFN) reported in a patient in arm B led to death. The primary endpoint of the study was undetectable HDV RNA at Week 24 after EOT. Main secondary endpoints included HDV RNA decline by ≥2 log10 IU/mL, ALT normalization and the combined response undetectable HDV RNA or decrease by ≥2 log10 IU/mL from BL and ALT normalization as well as HBsAg decrease.
The efficacy results are shown in table6. Figure 10 shows the decline in HDV RNA over time for the different treatment arms, respectively.
Table 6: Efficacy outcome from the MYR204 study
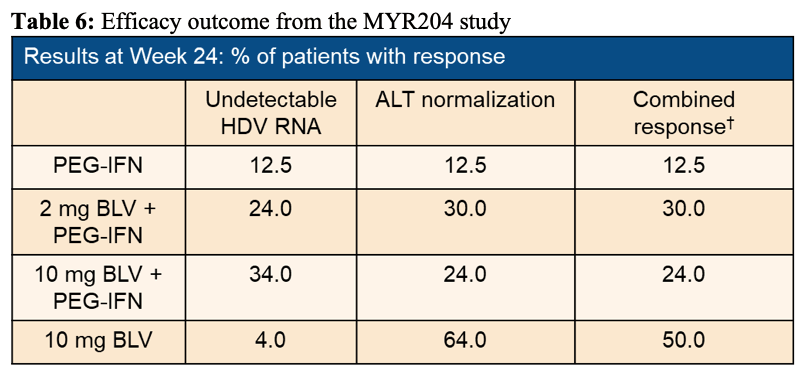
Figure 10: Decline in HDV RNA over time
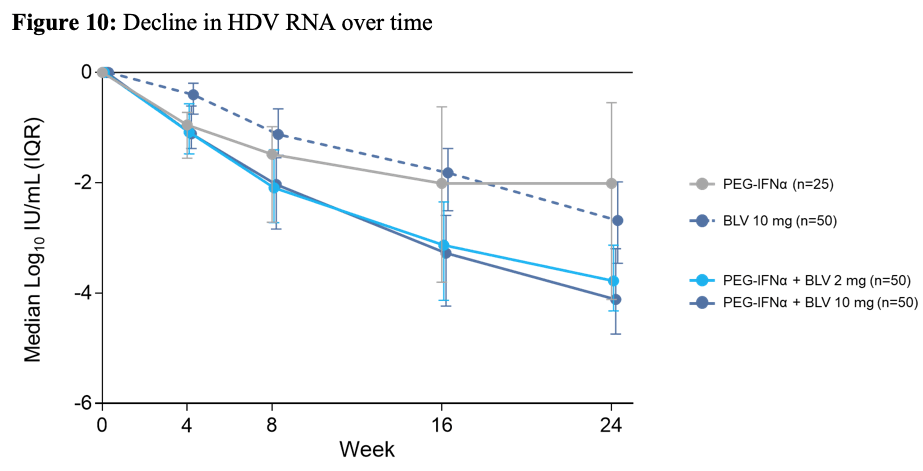
Combination of BLV with PEG-IFN resulted in higher rates of on-treatment undetectable HDV RNA, while higher rates of ALT normalization were observed with BLV monotherapy. No HBsAg loss was observed after 24 weeks of treatment. Modest declines in HBV DNA however, were observed in BLV containing arms compared to PEG-IFNα alone.
In conclusion, this new HDV therapy appears to be well tolerated. Combined response rates (2-log10 decline or undetectable HDV RNA with ALT normalization) were higher in BLV-treated arms compared to Peg-IFN alone with the highest biochemical response seen in BLV monotherapy arm. The questions, which remain are obviously how long should treatment with BLV last and will there be an improvement of liver disease related events over time, beyond changes in lab values such as liver enzyme normalization. And is there any value in adding PEG-IFN which does contribute to more adverse events over time.
Additional interim 24 week results for Bulevirtide were presented from the MYR301 study (15). This study evaluates the safety and efficacy of BLV administered subcutaneously at a dose of 2 or 10 mg qd for treatment of chronic HDV compared with no treatment for up to 144 weeks. The study design is shown below in figure 11.
Figure 11: Study design of the MYR301 study.
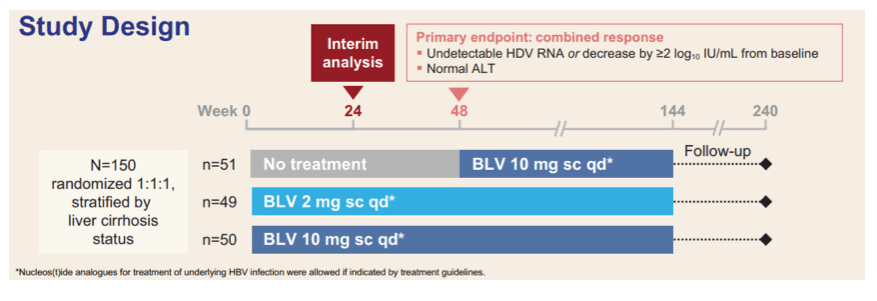
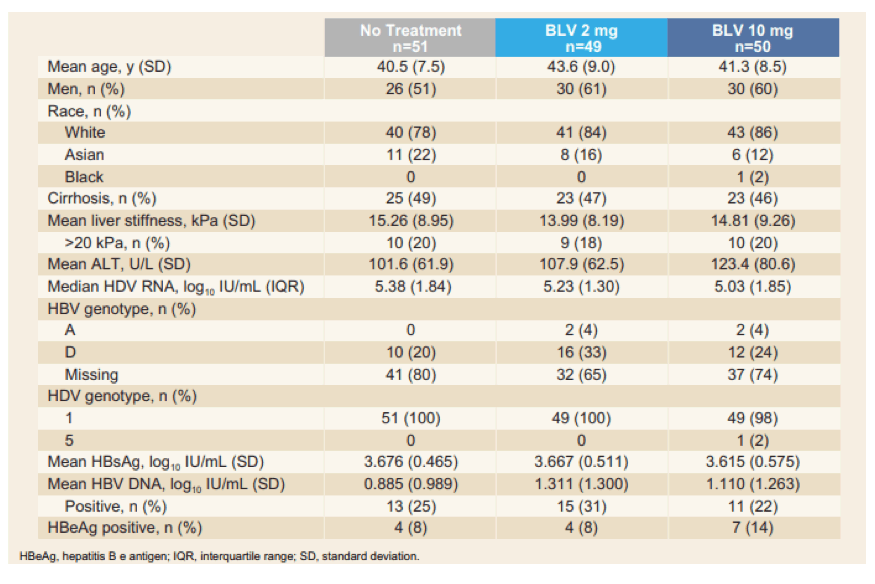
Adults with chronic HDV with or without cirrhosis, 1x < ALT < 10x upper limit of normal and Child-Pugh-Turcotte (CPT) ≤ 7 were enrolled into the study. The table 7 summarizes the main baseline characteristics of the study population.
Table 7: Baseline characteristics of the study population
Patients with controlled HIV coinfection were not excluded from the study. The primary study endpoint was a combined response of an undetectable HDV RNA or decrease by ≥2 log10 IU/mL from baseline and ALT normalization. The virologic and biochemical responses at Week 24 are shown in figure 12.
Figure 12: Study endpoints at week 24
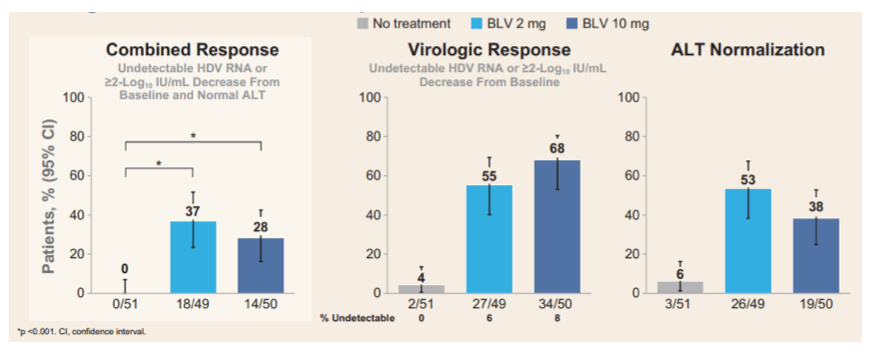
There were no serious AEs related to BLV or adverse events leading to discontinuation of study drug. 24 weeks of treatment with BLV was associated with significant HDV RNA declines and improvements in biochemical disease activity. Indeed, both BLV 2 and 10 mg, had significantly superior responses to the no treatment arm. Moreover, BLV 2 mg for 24 weeks had a numerically higher combined response rate than the BLV 10 mg dose. Again this study demonstrates clear antiviral effects of BLV. The question about duration of therapy and long-term clinical benefit however, remains equally unanswered. The final results after 144 weeks of treatment and subsequent additional 96 weeks of follow-up will allow additional important insights into this new treatment modality for HDV-infection.
SVR after all oral DAA therapy: who remains at risk for liver disease related events?
Direct acting antivirals (DAA) against hepatitis C improve the short-term outcome of patients with chronic hepatitis C. The long-term clinical course after interferon-free treatment of chronic hepatitis C however, has rarely been studied in large real-world cohorts. At this year ILC meeting data on 10,448 HCV patients being followed for up to 7 years after DAA therapy from the German Hepatitis C-Registry (DHC-R) were presented (16). The DHC-R is an ongoing, non-interventional, multicenter, prospective registry study. Data were collected between February 2014 and 1st January 2021 from more than 250 sites in Germany. The study flow is shown in figure 13. About 1/3 (32%) of the patients had liver cirrhosis at baseline. 26% (2,712/10,448) had a follow up of at least three years after end of treatment. Antiviral treatment containing ribavirin (RBV) were given to 2,359 patients, while 8,089 patients received a DAA regimen without RBV. The overall sustained virological response rates were 95% (9,951/10,448) and 97% (9,824/10,157) in intention-to-treat and per protocol analysis, respectively. During long-term follow-up, 181 patients died. Liver-related clinical events (liver transplantation, hepatocellular carcinoma (HCC), hepatic decompensation or increase in MELD score by ≥3 points) occurred in 416 (5.7%) patients. Figure 14 depicts the long-term risk of liver related complications in hepatitis C virus patients after antiviral therapy.
Figure 13: Study Flow
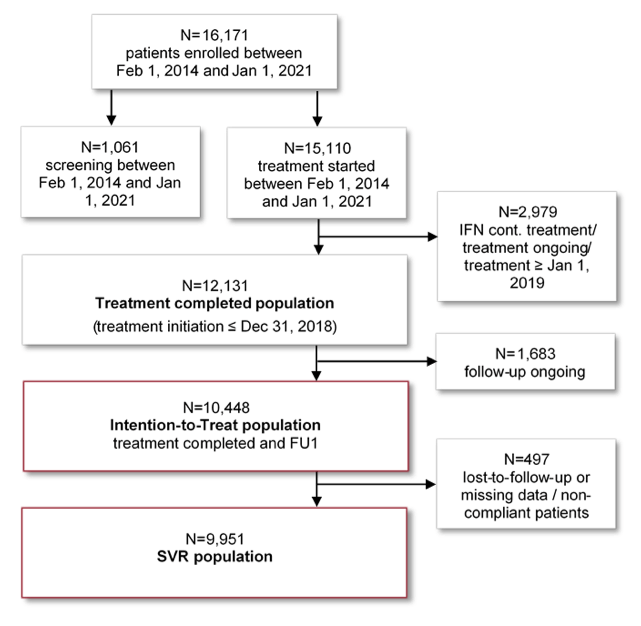
Figure 14: Kaplan Meier curve analysis of liver-related endpoint-free survival of patients achieving sustained virological response (SVR)
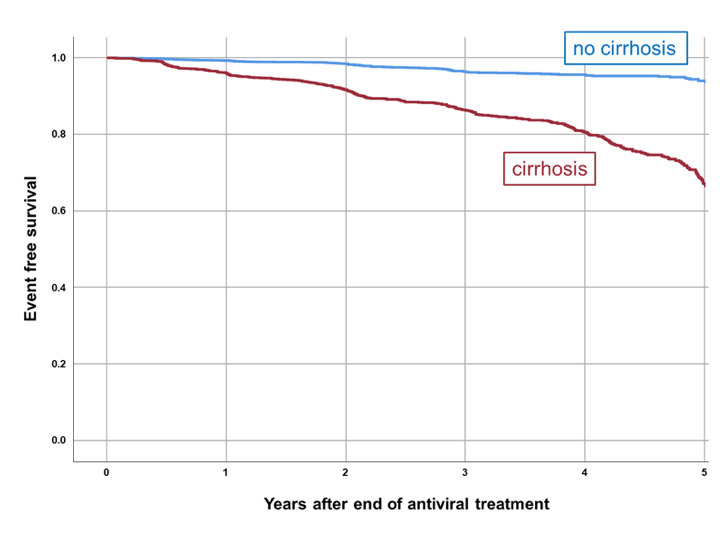
The overall annual incidence of de novo HCC was 0.3% (SVR ITT patients). The annual post-treatment HCC risk in SVR ITT patients with liver cirrhosis at years 1-2 was 0.9% and 0.5% at year 3-5, respectively. Baseline parameters associated with liver-related endpoints (defined as liver transplantation, hepatic decompensation or increase in MELD score by ≥3 points) in patients achieving SVR were treatment experience (OR=1.259), male sex (OR=0.328), low hemoglobin level (OR=0.821), mildly decreased eGFR (OR=0.985), and elevated alkaline phosphatase (OR=1.007). Baseline parameters associated with de novo HCC development in patients achieving SVR were only presence of liver cirrhosis (OR=4.232) and treatment experience (OR=1.420).
In conclusion, patients with chronic hepatitis C and liver cirrhosis remain at risk of complications of liver disease >3 years after cure. Continued long-term monitoring is strongly recommended in these patients.
At the ILC conference an Italian study group also examined the potential long-term benefits of SVR in 647 HCV cirrhotics (17). They assessed the SVR impact on liver-related (LRE), non-liver related events (NLRE) and mortality in cirrhotics successfully treated with DAA therapy. In their study consecutive SVR cirrhotics were enrolled in a longitudinal, single-center study, and divided into Cohort A (Child-Pugh-Turcotte (CPT)-A without previous LRE), Cohort B (previous non-HCC LRE), and Cohort C (previous HCC). Patients were followed for a median of 51 (8-68) months. The median age was 65 years and 58% of the participants were male and 89% had cirrhosis classified as CPT stage A. Overall, 50 patients died, 42% liver-related and 58% non-liver related. 14 (2%) patients underwent liver transplantation during the observation period. HCC was the most frequent cause of liver-related death and liver transplantation (LT). The 5-year cumulative incidence for the combined endpoint liver-related death and LT was 7.7%. The overall LREs 5-year cumulative incidences by cohort are shown in table 8.
Table 8: LREs 5-year cumulative incidences
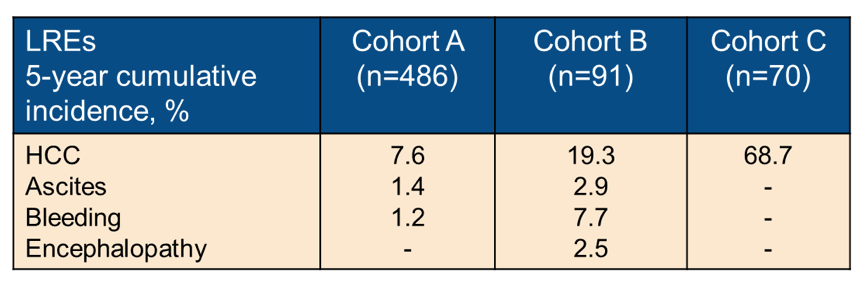
In conclusion, despite HCV cure, patients with cirrhosis face a significant residual risk of HCC and non-liver-related complications.
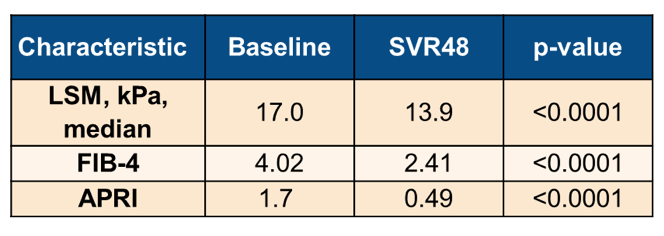
The association between non-invasive tests for fibrosis and liver-related events (LREs) after a sustained virological response (SVR) to direct-acting antivirals (DAA) in HCV cirrhotics remains undefined. Indeed the EASL HCV guidelines from 2020 point out that notably, non-invasive tools should not be used to assess fibrosis stage after therapy, as they are unreliable in this setting (18). At this year ILC a study was presented which investigated changes in non-invasive tests for fibrosis following DAA therapy and the association with liver disease related events (LREs) in a large cohort of patients with cirrhosis (19). Consecutive patients with compensated cirrhosis achieving SVR following HCV treatment between 2014 and 2019 in a single centre (Milan) were enrolled. All liver disease related events were recorded including HCC, ascites, variceal bleeding, and encephalopathy. LREs occurring before SVR were excluded. Liver stiffness measurements (LSM), Fibrosis-4 (FIB-4) and AST to Platelets Ratio index (APRI) were assessed at DAA start (baseline) and 12 months post-treatment (SVR48). 486 patients were analyzed with a median age of 64 (24-92) years, 59% males, and 19% diabetics. Compared with baseline, all non-invasive tests significantly declined
at SVR48. Table 9 summarizes the main results from this observational cohort.
Table 9: Results of LSM, FIB-4 and APRI in HCV patients with cirrhosis at baseline and SVR48 after DAA therapy.
After 51 (8-68) months from baseline, 41 (8%) patients developed an LRE (32 HCC, 6 ascites, 3 bleedings). The 5-year cumulative incidence of a LRE was 10.2% (95% CI 7-13%). By multivariate analysis, neither baseline nor SVR48 APRI, FIB-4, ΔAPRI, or ΔFIB-4 were associated with LREs. Conversely, both baseline (HR 1.05, p=0.01) and SVR48 (HR 1.02, p=0.003) LSM, but not ΔLSM, independently predicted LREs, together with diabetes, anti-HBc, and male sex. By ROC curve analysis, LSM at baseline >20.8 and at SVR48 >16.1 kPa were the optimal cut-offs for LREs prediction. The 5-year cumulative incidence of LREs was 16.3% vs 5.5% in patients with baseline LSM > or ≤20.8 kPa, respectively (p=0.01). The 5-year cumulative incidence of LRE was 21% vs 3.7% for SVR48 LSM > or ≤16.1 kPa, respectively (p=0.003).
In summary, in this large cohort of patients with compensated HCV cirrhosis achieving SVR to DAAs, LSM but not FIB-4 or APRI score was associated with LREs. Of note, it was not the change in LSM after SVR but the actual value obtained at baseline or at SVR48 which was predictive. This implies that at least LSM values may be helpful throughout different liver disease stages to predict risk for liver disease related events.
HCV elimination: success and challenges
The Global Health Sector Strategy (GHSS) on viral hepatitis, approved by the World Health Assembly in 2016, has the vision of a world where transmission of viral hepatitis is halted and everyone living with viral hepatitis has access to safe, affordable and effective prevention, diagnosis, care and treatment. Its goal is to eliminate viral hepatitis as a major public health problem by 2030. The 2030 GHSS relative reduction reference targets for hepatitis B and C are a reduction in HBV and HCV incidence by 95% and 80%, respectively and a reduction in HBV and HCV-related mortality by 65%. New WHO Guidance for country validation of viral hepatitis B and C elimination was released during a joint EASL-CDC-ECDC and WHO symposium "Viral Hepatitis Elimination - Assessing the progress in 2021" at the EASL International Liver Congress 2021 (20). This represents the first-ever global guidance for countries seeking to validate elimination of hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infection as a public health problem.
In April 2015, with the technical assistance of U.S. CDC and commitment from Gilead Sciences to donate direct acting antivirals (DAAs), Georgia launched the world's first HCV elimination program. Key strategies included nationwide HCV screening, active case finding, linkage to care, decentralized care, provision of treatment for all HCV persons, and effective prevention interventions. The initial goal of the program was to achieve the following targets by 2020: a) diagnose 90% of HCV-infected persons, b) treat 95% of those diagnosed, and c) cure 95% of those treated. At this year ILC 2021 an update of the elimination program which now has completed year 5 was provided (21). Data on persons tested for chronic HCV infection, and those deemed cured by SVR were extracted as of 31 December 2020. Treatment was provided with Sofosbuvir, Ledipasvir/Sofosbuvir or Velpatasvir/Sofosbuvir-based regimens. The corresponding cascade of care data for this time-point is depicted in figure 15.
Figure 15: HCV care cascade as of 31st December 2021.
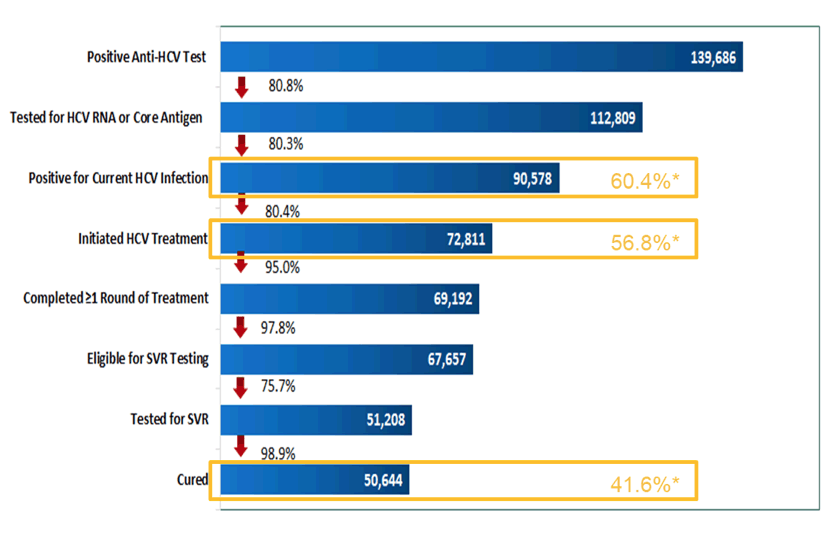
High cure rates were achieved for all HCV genotypes: 98.9% in genotype 1, 98.9% in genotype 2 and 98.3% in genotype 3. Clearly, these SVR rates are very impressive particular for genotype 3 which traditionally is the somewhat more difficult genotype to treat. Treatment effectiveness was comparable among persons with advanced fibrosis (F3 and F4) with 98.2% achieving SVR, and among patients with mild or no liver fibrosis (≤ F2), who had a SVR rate of 99.1%. Without doubt, considerable progress within the elimination program has been achieved, but with just over 40% cured there is still a long way to go to reach the self-imposed goal of treating 95% of those diagnosed with HCV in the country.
Concerted efforts to increase HCV treatment rates amongst PWIDs are required to eliminate HCV as a public health threat. Due to the close relationship between injecting drug use, incarceration and chronic HCV, the prevalence of HCV is up to 40 times greater within correctional facilities compared with the community. However, very few prisoners are treated for HCV while incarcerated. National Health Service England (NHSE) plans to eliminate Hepatitis C in England by 2025, five years earlier than World Health Organization goals. At this year ILC data was presented from a UK study on "High Intensive Test and Treat (HITT) Interventions" in the prison system (22). Through a collaboration between The Hepatitis C Trust (HCT), Her Majesty's Prison and Probation Service, Prison Healthcare provider Practice Plus Group, Gilead Sciences and NHS England, a process was developed to screen entire prisons for HCV over 1-5 days and rapidly initiate treatment. Peers from the Hepatitis C Trust engaged with non-medical staff and residents in advance of, and during, the HITT to raise awareness and reduce stigma. HCV screening was performed using rapid point-of-care antibody tests followed by PCR-based assessment of patients positive for HCV antibodies. Residents were encouraged to engage using incentives such as chocolates, toiletries and telephone credit. The patient care pathway for 2020 HITTs is shown in figure 16.
Figure 16: Patient care pathway
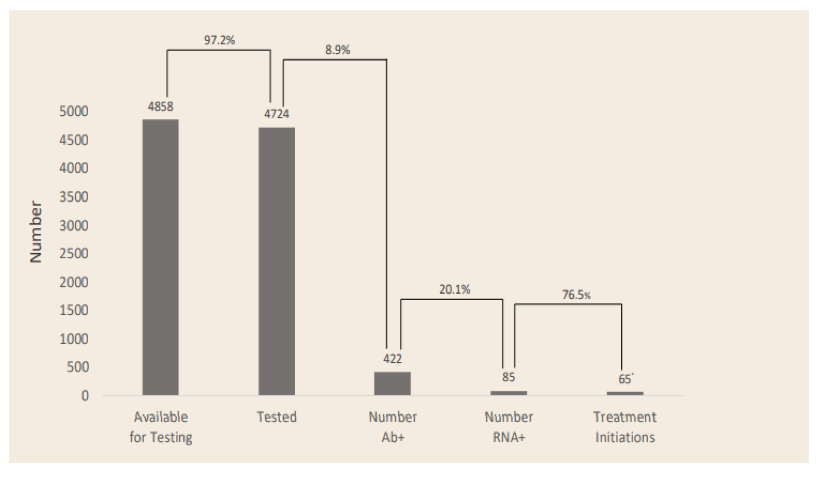
* COVID-19 impacted prisons following the national lockdown, which affected the initiation of treatment following 3 HITTs where testing took place in March 2020.
The use of the HITT model to test for HCV in over 95% of a prison residents, and rapidly initiate direct-acting antiviral therapy in those testing positive, has been shown to be highly effective in working towards HCV micro-elimination of individual prisons despite some delays through the COVID-19 pandemic. This model clearly supports broader dissemination on a more global level. Even higher rates of HCV-RNA positive prisoners can be expected in prison settings where HCV screening at entry is far less standardized and implemented as general intervention.
What was new for NAFLD and NASH?
First of all great guidance was provided by EASL in form of a new set of practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis. Clearly, limitations for non-invasive methods exist but non-invasive scores, serum markers, liver stiffness and imaging methods can help to identify advanced fibrosis in patients at risk from low-prevalence populations significantly better than clinical acumen alone (1). Non-invasive tests (NITs) should also provide prognostic information beyond fibrosis stage and allow for monitoring of liver fibrosis and its complications. Therefore, the EASL guidelines strongly recommend that individuals at risk of advanced fibrosis due to metabolic risk factors and/or harmful use of alcohol should be entered into appropriate risk stratification pathways using non-invasive fibrosis tests (1). The selection of non-invasive NITs and the design of diagnostic pathways for testing low-prevalence populations for advanced fibrosis should be performed in consultation with a liver specialist (1). Figure 17 shows the algorithm suggested by EASL for use of NITs in patients observed in primary care or outside the liver clinic.
Figure 17: Proposed use of NITs in patients observed in primary care or outside the liver clinic (1).
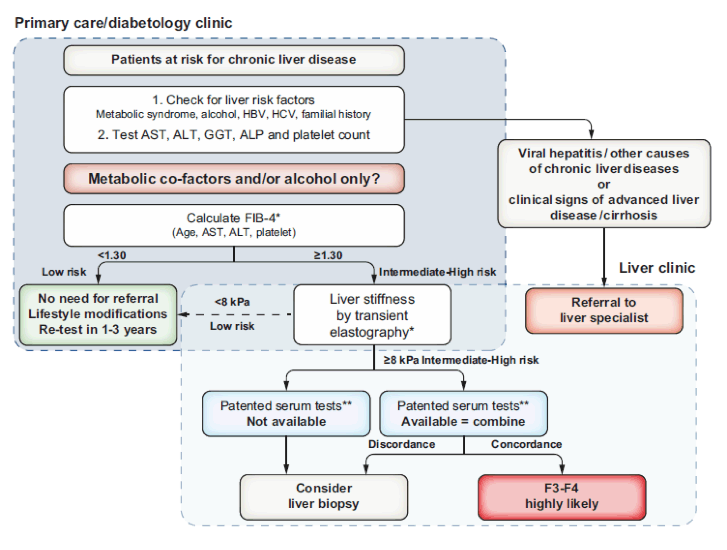
*Transient elastography or FIB-4may be performed before or after referral to liver specialist according to local availability and pathways. **Cut-offs to use: ELFTM 9.8 (NAFLD/ALD); FibroMeter 0.45 (NAFLD), Fibrotest 0.48 (NAFLD). ALD, alcoholrelated liver disease; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ELF, enhanced liver fibrosis; FIB-4, fibrosis-4; NAFLD, non-alcoholic fatty liver disease.
As shown in the algorithm, FIB-4 can be used in patients with metabolic co-factors and/or alcoholic liver disease to identify patients requiring referral to the specialist liver clinic. Indeed, the FIB-4 index, consisting of four parameters (age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelets), is a simple, cheap, and accurate tool to help identify patients at risk for chronic liver disease. The algorithm provided is very helpful for colleagues who see a lot of patients with increased risk for metabolic liver disease (for example ID doctors managing HIV patients) to screen there at risk populations accordingly.
Hyperferritinemia is common in patients with Non-Alcoholic Fatty Liver Disease (NAFLD) and correlates with the severity of liver fibrosis. At this year ILC meeting data was presented on the impact of ferritin on long-term outcomes and survival in a large cohort of NAFLD patients (22a). Overall, 1,247 individuals with biopsy-proven NAFLD from tertiary centres in Italy, Spain, UK, and Australia were included into the study. Clinical and biochemical data was collected at time of liver biopsy. Ferritin levels of 300 μg/L (men) and 200 μg/L (women) were considered as the ULN. Clinical outcomes were assessed after a median follow-up of 90 months including all liver-related events such as ascites, encephalopathy, variceal bleeding and hepatocellular carcinoma as well as overall survival. Main baseline characteristics as well as clinical outcomes after 90 months follow-up are listed in table 10.
Table 10: Results: Baseline characteristics and outcome findings.
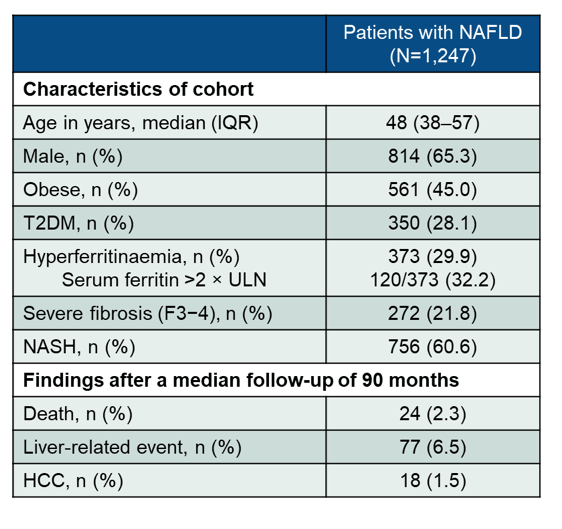
Most interestingly, serum ferritin >2 X ULN was significantly associated with F3-4 fibrosis OR 1.90 (95% CI, 1.19-3.03; p=0.007). In univariate analysis liver-related events and mortality were significantly higher in patients with ferritin 2 X ULN (log-rank test: p=0.004 and p=0.001, respectively). In multivariate analysis (cox regression adjusted for age, body mass index, diabetes, and fibrosis) ferritin 2X ULN predicted mortality (aHR 3.04; 95% CI, 1.16-7.93; p=0.023), but not liver-related events (aHR 1.67; 95% CI, 0.90-3.11; p=0.105).
The authors conclude that ferritin levels higher than 2 X ULN are associated with
severe liver fibrosis in NAFLD patients and predict long-term mortality.
Numerous new potential agents and targets for treatment of NAFLD/NASH were presented during the ILC meeting. A detailed description of all presented studies are beyond the scope of this summary. A few selected studies will be summarized below.
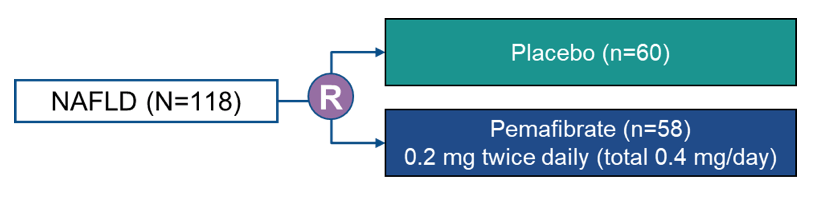
Pemafibrate is a novel selective peroxisome proliferator-activated receptor alpha modulator (SPPARM alpha), which has been approved for treatment of dyslidemia in Japan (23). Pemafibrate reportedly improved nonalcoholic steatohepatitis (NASH) in the mice model as well as serum liver parameters in patients with dyslipidemia. At ILC 2021 the results of a randomized, double-blind, placebo-controlled study were presented which assessed the efficacy and safety of pemafibrate in patients with nonalcoholic fatty disease (NAFLD). Key inclusion criteria were liver fat content of ≥ 10 %, by magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF), liver stiffness of ≥ 2.5 kPa by magnetic resonance elastography (MRE) and elevated alanine aminotransferase (ALT) level. The patients were randomized to either pemafibrate (0.4 mg/day, BID) or placebo. The corresponding study design is shown in figure 18. The primary endpoint of the study was percent change in liver fat content from baseline to week 24, and the key secondary endpoints were percent changes in liver stiffness and ALT level from baseline to week 72 and 24, respectively.
Figure 18: study design
Pemafibrate therapy did not significantly alter liver fat content, however, liver stiffness and ALT level significantly decreased in pemafibrate compared to placebo (see figure 19a and b). Additionally, pemafibrate significantly improved lipid parameters (e.g., triglycerides, LDL-C). Pemafibrate showed no significant safety issues and it did not affect renal functions (e.g., serum creatinine).
Figure 19a: Change in MRE-based liver stiffness over 72 weeks
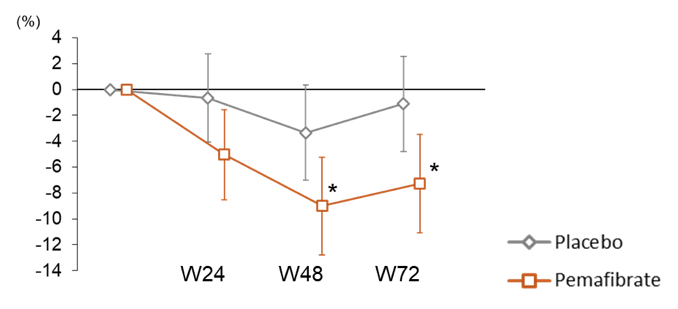
Figure 17b: Change in ALT levels over 72 weeks
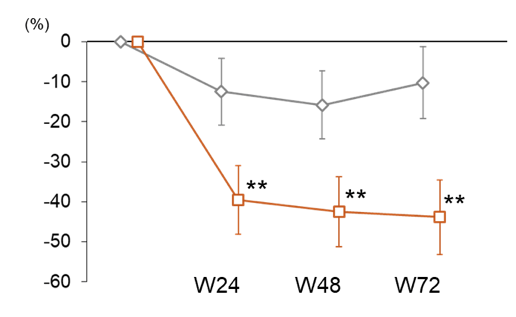
The authors concluded that pemafibrate improves liver stiffness by MRE and serum liver and lipid parameters despite not decreasing liver fat content. These results suggest that pemafibrate has potential to be further explored and developed for therapeutic use in NAFLD/NASH.
The renin-angiotensin-aldosterone system has been reported to be associated with inflammation and fibrosis in NAFLD (24). Moreover, renin-angiotensin system blockers, including angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), have been shown to exert protective effects against liver fibrosis (25). These effects are due to suppression of hepatic stellate cell transformation into hepatic myofibroblasts (HMs) in response to elevated expression of pro-inflammatory cytokines and reduced expression of tissue growth factors. The clinical value of these agents in patients with NAFLD however remains uncertain. At ILC 2021 the results from a very large retrospective cohort investigating the impact of ACEI/ARB use on the risk of hepatocellular carcinoma (HCC) and cirrhotic complications in a territory-wide cohort of NAFLD patients was presented (26). All NAFLD patients aged 18 years or above between January 1, 2000 and December 31, 2014 were included. A propensity score-weighted Cox proportional hazards model was used to balance the clinical characteristics between ACEI/ARB users and non-users. Fine-Gray model was used to adjust for competing risk of death. Data from an impressive 12,494 NAFLD patients was analyzed. Mean age was 54.4±14.5 years and 50.4% of the cohort were male (6,301 men). Overall, 7,428 (59.5%) received ACEI/ARB and 5,066 (40.5%) did not. Compared to non-users, ACEI/ARB users were older, more likely to have type 2 diabetes, hypertension and cirrhosis, and had worse renal function. At a median (interquartile range) follow-up of 9.9 (5.4-14.2) years, 268 (3.6%) ACEI/ARB users and 173 (3.4%) non-users developed liver-related events (a composite endpoint of HCC and cirrhotic complications). After propensity score weighting, ACEI/ARB treatment was associated with a lower risk of liver-related events, HCC and cirrhotic complications, which are summarized in table 11.
Table 11: Results from the propensity score-weighted Cox proportional hazards model.
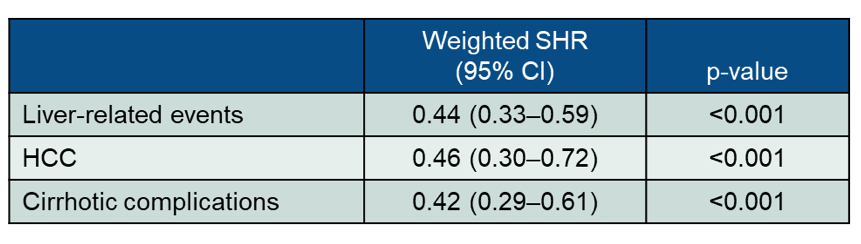
In conclusion, ACEI/ARB treatment was associated with a lower risk of liver-related events, HCC and cirrhotic complications in NAFLD patients. As ACEI/ARB are widely available this allows a preferential use of these agents at least in patients with concomitant arterial hypertension and NAFLD.
Another NAFLD treatment study which received wide attention was a randomized clinical trial from Sweden evaluating the effects of intermittent calorie restriction, low-carb high-fat diet against standard dietary advice (27, 28). Currently, there is no approved pharmacological treatment for NAFLD, although several compounds are under development. Instead, lifestyle changes leading to weight reduction are considered first-line treatment. Indeed, weight reduction has the potential to reverse steatosis, non-alcoholic steatohepatitis (NASH), and liver fibrosis (29). Figure 18 depicts the study flow of this 12-week randomized controlled trial comparing treatment for NAFLD with LCHF and 5:2 diets administered by a dietitian, and standard of care (SoC) recommendations given by a physician. Magnetic resonance spectroscopy (MRS) was used to measure the reduction of liver steatosis from baseline to end of treatment (EoT).
Figure 18: Study flow
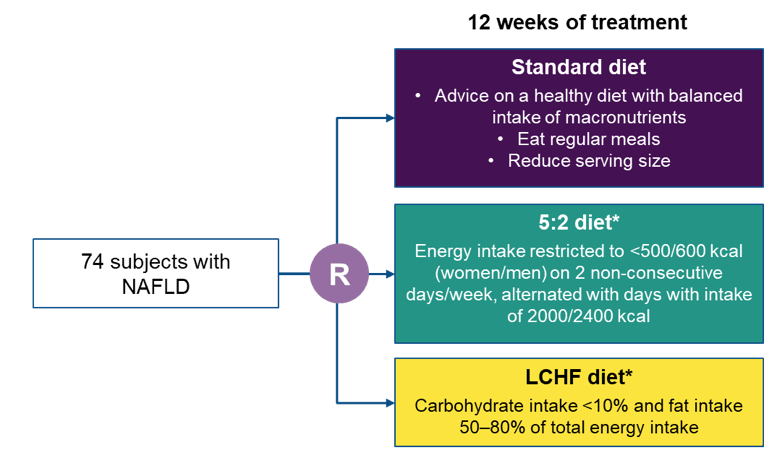
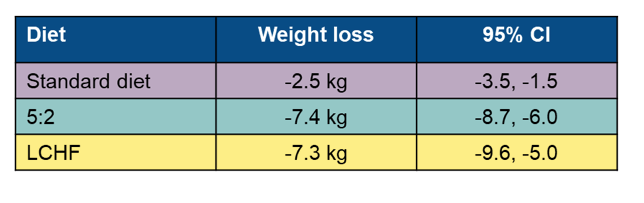
Inclusion criteria were 1 of either: (1) NAFLD diagnosed by radiologic assessment (ultrasound, computed tomography [CT] or magnetic resonance imaging), (2) Fibroscan® (Echosens, Paris, France) with a controlled attenuation parameter (CAP) >280 dB/m in combination with obesity (BMI ≥30 kg/m2), or (3) CAP >280 and elevated alanine aminotransferase (ALT) (>46 IU/L for women, >66 IU/L for men) and overweight (BMI ≥25 kg/m2). Overall 74 subjects with NAFLD were included. LCHF and 5:2 diets were equally effective in reducing steatosis (p=0.41) and body weight (p=0.78) and both were more effective than the standard diet (see also figure 19 and table 12).
Figure 19: Relative change in hepatic steatosis as measured with MR Spectroscopy
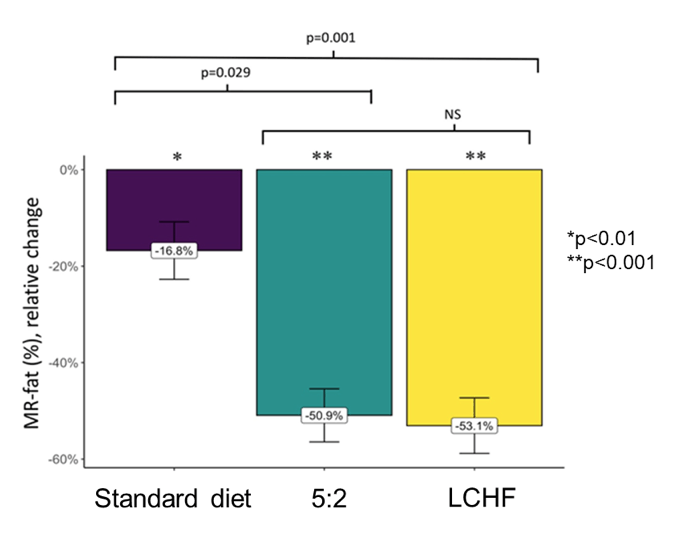
Table 12: Change in body weight
Liver stiffness improved with the 5:2 diet and SoC, but not the LCHF diet: LCHF diet: -0.3 kPa (p=0.52), 5:2 diet: -1.8 kPa (p<0.001) and standard diet: -1.5 kPa (p=0.005), respectively. LDL levels were significantly reduced with the 5:2 diet (-0.4 mmol/L, p<0.001), while a trend towards a higher LDL was observed with the LCHF diet (+0.2 mmol/L, p=0.075).
In summary, this randomized controlled trial shows that a dietitian-supported treatment with a LCHF or 5:2 diet was more effective than SoC in reducing liver fat after a 12-week treatment. No difference in liver fat reduction was observed between the 5:2 and the LCHF diet. Previous studies have reported a correlation between weight loss and reduction of liver fat in NAFLD. The results from this study further support this correlation and demonstrate that dietary advice can be individualized to achieve short-term weight loss. Individualized dietary counselling may be crucial considering that many person-specific factors may affect body metabolism as well.
COVID-19 and chronic liver disease: what needs to be considered?
Older adults and people of any age who have serious underlying medical conditions, including people with liver disease, might be at higher risk for severe illness from COVID-19. At this year ILC meeting data on the outcome of COVID-19 disease in patients with chronic liver disease was presented from France (30, 31). The data source was the French National Hospital Discharge database (Programme de Médicalisation des Systèmes d'Information), which contains all public and private claims for acute inpatient/day case hospital admissions and post-acute care since 2011. The outcomes of all Covid-19 adult inpatients in France from 2020 were explored including back-tracing of the hospital trajectory of each selected patient and documentation of all previous/underlying conditions. Adjusted odds-ratios were computed to measure the associations between chronic liver disease, alcohol use disorders, mechanical ventilation and day-30, in-hospital, mortality. The sample comprised 259,110 patients [median (interquartile range) age 70 (54, 83) years; 52% men], including 15,746 (6.0%) and 10,006 (3.9%) patients with chronic liver disease and alcohol use disorders, respectively. Mortality was 38,203 (15%) patients, including 7,475 (28%) after mechanical ventilation, and 2,941 (19%) with chronic liver disease. The adjusted odds-ratios for invasive mechanical ventilation and day-30 mortality are shown in figure 20.
Figure 20: Multivariate risk of mechanical ventilation and day-30 in-hospital mortality after COVID-19 in France (n=259,110).
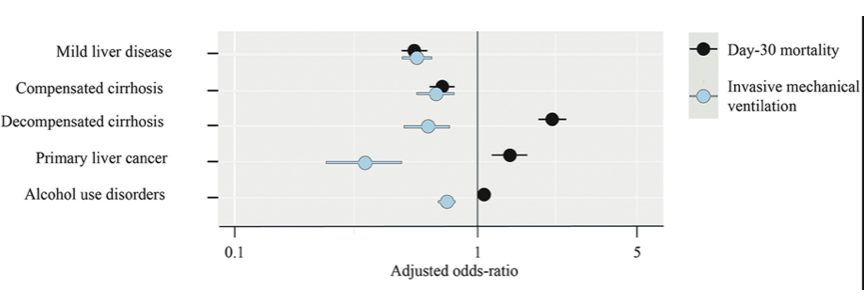
Chronic liver disease increased the risk of Covid-19 in-hospital death in France in 2020. Therapeutic effort limitation may have contributed to Covid-19 death of patients with a liver-related complication or with alcohol use disorders.
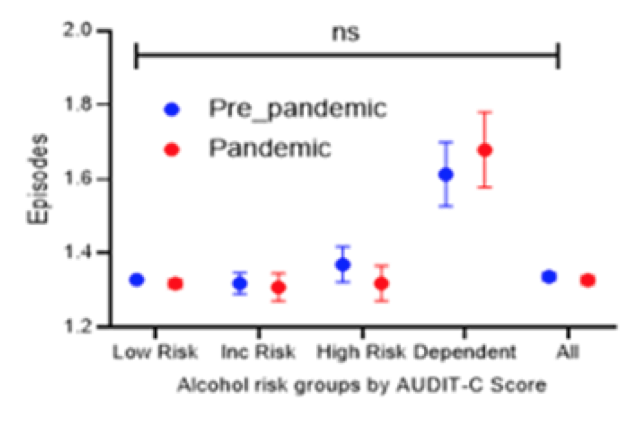
The Covid-19 pandemic has presented significant challenges to health care services. Hospitals have reported a two-fold increase in admissions due to alcohol-related liver disease. Patients are sicker, and higher numbers are requiring high dependency care. More representative data on the impact of Covid-19 on alcohol use disorder (AUD) among hospitalized patients is lacking. Therefore, a study from the UK was presented at ILC which aimed at describing the epidemiology of alcohol use disorder during the pandemic among hospitalized patients and compare it with the Pre-pandemic cohort (33). Moreover, the investigators identified key demographic characteristics which can be used to risk-stratify individuals presenting to healthcare for targeted alcohol support services. Retrospective cohorts were defined as Pre-pandemic (Pre-Covid-19) (1st April to 31st October 2019) and Pandemic (Covid-19) (1st April to 31st October 2020) admitted to Nottingham University Hospital UK. All patients were offered alcohol assessment by AUDIT-C (The AUDIT-C is scored on a scale of 0-12 (scores of 0 reflect no alcohol use). In men, a score of 4 or more is considered positive; in women, a score of 3 or more is considered positive). In this study increased and high-risk alcohol use was defined as AUDIT-C 5-7, 8-10, and alcohol dependence 11-12. Variation in AUDIT-C was determined by age, sex, ethnicity and admission type/speciality. Patients admitted directly to intensive care were excluded. A total of n=20598 and n=27356 were included in Pandemic and Pre-pandemic cohorts, respectively. 18% of patients had alcohol use disorder. Overall, alcohol dependency was higher in the pandemic cohort. The admissions for the two cohorts by alcohol risk group is shown in figure 21.
Figure 21: Admissions stratified by alcohol risk group and cohort.
Moreover, patients in all 3 alcohol risk groups were significantly younger than in
the low-risk group (p<0.05). Male sex and white ethnicity were additionally associated with a higher prevalence of AUD. In the pandemic cohort there was a 16 times higher risk of mental and behavioral disorders in the alcohol-dependent group (OR 15.8; p<0.001). The Covid-19 positive patients with concomitant AUD had a longer hospital stay and died at a significantly younger age (mean difference 8 years, p<0.05). Emergency was the predominant mode of admission. In summary, the COVID-19 pandemic has had a clear impact on drinking behaviors with a higher proportion of those admitted during COVID-19 pandemic being alcohol-dependent and admitted as a medical emergency.
Pfizer's and Moderna's mRNA SARS-CoV-2 vaccines show high efficacy in clinical and real-world data. In Israel, more than 60% of ≥90 year old's population received both vaccine doses. Whether certain patient groups may have a higher risk for vaccine failure is still poorly understood. At this year ILC a researcher group from Israel aimed at assessing the impact of advanced chronic liver disease on the efficacy of Pfizer's SARS-CoV-2 vaccines (33). Indeed, rates of seroconversion after hepatitis B virus (HBV) immunization, and the durability of humoral immunity after pneumococcal and influenza vaccination are all markedly reduced in patients with liver cirrhosis (34-36). In this pilot study hepatic fibrosis (by Fib-4 or by liver biopsy) was correlated with vaccine responses in subjects who received both doses of BNT162b2 vaccine and tested for serum SARS-CoV-2 spike immunoglobulins (S IgG) at least 7 days later (Liaison assay). Overall, 501 NAFLD patients who underwent Fib-4 assessment and had Sars-Cov-2 vaccination as well as antibody assessment available were included into the study. Excellent vaccination responses (defined as serum IgG titers ≥200 AU/ml) were observed in 68.0% of patients with Fib-4 <1.3, in 56.9% of patients with Fib-4 1.3-2 and only 44.2% of patients with Fib-4 >2. A further substudy in 140 patients with NAFLD and histologies available showed high overall vaccine responses of 98.2%. Lower antibody titers however, were seen in NAFLD patients with more advanced fibrosis upon histologic examination. Vaccination responses in liver transplantation patients under immunesuppressive therapy were considerably lower than in the other patient groups. 32/90 OLT patients after post-OLT vaccination showed anti-S IgG<19. 5 OLT patients developed COVID-19, one case after the first vaccination shot and 4 cases after the 2nd shot. In summary, there were therefore 41.1% vaccination failures (5 COVID-19 cases and 32 with no development of antibodies.
In conclusion, overall vaccine response rate in NAFLD patients was high but affected by age, immune status and fibrosis stage.
Summary
• Type of hepatitis B treatment (ETV or TDF) does not significantly impact HBsAg loss rate after statistical adjustment. Virological and clinical relapse however, are observed earlier and more frequently in patients treated with TDF versus ETV, with all measured variables balanced. Virological relapse rates in the two groups become comparable after first year off-therapy and retreatment rates do not differ between the treatment groups.
• The effects of TAF treatment for highly viremic HBV-infected pregnant women are comparable with TDF treatment in terms of maternal HBV DNA reduction and in preventing mother-to- infant transmission.
• TAF therapy initiated during the second trimester for HBV-infected pregnant women with positive HBeAg and high HBV DNA level was effective in preventing MTCT, and there were no safety concerns for mothers and infants with 28 weeks of follow up after delivery. The long-term benefits of using TAF in MTCT, when compared with TDF, particularly on bone and renal safety, remain to be explored.
• An HBIg-free strategy with maternal use of TDF and early HBV vaccination at birth could be effective to reduce HBV MTCT, specifically if TDF is administered for ≥1 month prior delivery. For women with low-level hepatitis B viremia, an early vaccination without HBIg could be enough to reduce HBV MTCT.
• Patients with chronic hepatitis B without nucleos(t)tide analogue treatment have a higher risk of extrahepatic malignancy, which can be attenuated with HBV nucleos(t)ide treatment.
• Moderate-severe renally-impaired CHB patients as well as end-stage renal disease patients on hemodialysis, who were switched to TAF from TDF and/or other HBV antivirals maintained high rates of hepatitis B viral suppression, and bone and renal parameters remained stable or slightly improved after 2 years of treatment.
• AB-729 is a RNA interference therapeutic that blocks all HBV RNA transcripts. Robust mean declines in HBsAg were sustained with repeat dosing of AB-729, with most subjects reaching HBsAg<100 IU/mL and no meaningful differences observed to date between dose and/or dosing intervals. Repeat dosing with AB-729 was generally safe and well toleratedGSK3228836 (GSK836) is a modified antisense oligonucleotide which triggers multiple immunological responses in responders experiencing decline in HBsAg levels and ALT elevations. The IFN-gamma pathway appears to be central to mediating immunological responses during HBsAg decline leading to ALT elevation in responders.
• Bulevirtide monotherapy or combination with PEG-IFNα-2a was safe and well tolerated through 24 weeks of therapy. Combination of BLV with PEG-IFN resulted in higher rates of on-treatment undetectable HDV RNA, while higher rates of ALT normalization were observed with BLV monotherapy.
• 24 weeks of treatment with BLV mono-therapy was associated with significant HDV RNA declines and improvements in biochemical disease activity. Longer follow-up is needed to better define the clinical benefit of this new HDV therapy.
• Patients with chronic hepatitis C and liver cirrhosis remain at risk of complications of liver disease >3 years after cure. Continued long-term monitoring is strongly recommended in these patients.
• In patients with compensated HCV cirrhosis achieving SVR to DAAs, LSM but not FIB-4 or APRI score are associated with LREs.
• Considerable progress within the Georgian elimination program has been achieved, but with just over 40% cured there is still a long way to go to reach the self-imposed goal of treating 95% of those diagnosed with HCV in the country.
• The use of the "High Intensive Test and Treat (HITT) model to test for HCV in over 95% of a prison residents, and rapidly initiate direct-acting antiviral therapy in those testing positive, has been demonstrated to be highly effective in working towards HCV micro-elimination of individual prisons.
• The non-invasive fibrosis score FIB-4 can be used in patients with metabolic co-factors and/or alcoholic liver disease to identify patients requiring referral to the specialist liver clinic.
• Ferritin levels higher than 2 X ULN are associated with severe liver fibrosis in NAFLD patients and predict long-term mortality.
• Pemafibrate in a proof-of-concept trial improved liver stiffness by MRE and serum liver and lipid parameters despite not decreasing liver fat content. These results suggest that pemafibrate potentially has some promise as a therapeutic agent for NAFLD/NASH.
• Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) treatment is associated with a lower risk of liver-related events, HCC and cirrhotic complications in NAFLD patients.
• Low-Carb High-Fat and 5:2 intermittant calorie restriction diets are more effective than standard dietary advice in reducing steatosis and body weight in NAFLD, suggesting dietary advice can be adjusted according to preference.
• Chronic liver disease increased the risk of Covid-19 death in France in 2020. The results from this large cohort study suggest that a limitation of the therapeutic effort may have contributed to the excess of mortality of patients with a liver-related complication and of patients with alcohol use disorders in France in 2020.
• The COVID-19 pandemic has had a clear impact on drinking behaviours with a higher proportion of those admitted during COVID-19 pandemic being alcohol-dependent and admitted as a medical emergency.
• Older age as well as advanced fibrosis with decreased steatosis are risk factors for lower vaccine response for Pfizer's BNT162b2 vaccine. A third dose vaccine booster should be evaluated in these vulnerable patient populations.
References
1. European Association for the Study of the Liver; List of panel members (alphabetical order), Berzigotti A, Boursier J, Castera L, Cazzagon N, Friedrich-Rust M, Petta S, Thiele M, Tsochatzis E. Easl Clinical Practice Guidelines (Cpgs) On Non-Invasive Tests For Evaluation Of Liver Disease Severity And Prognosis- 2020 Update. J Hepatol. 2021:S0168-8278(21)00398-6. Epub ahead of print.
2. Su TH et al. Distinct relapse rates and risk predictors after discontinuing tenofovir and entecavir therapy. J Infect Dis. 2018;217:1193-1201.
3. Choi HJS et al. Earlier and more frequent virological and clinical relapse after cessation of tenofovir disoproxil fumarate versus entecavir therapy: Results from a large, global, multi-ethnic cohort of chronic hepatitis B patients (RETRACT-B study). ILC 2021;PO-1841
4. Chen HL et al. Effects of tenofovir alafenamide fumarate treatment in pregnant women on maternal viral load reduction and preventing mother-to-infant HBV transmission. ILC 2021; PO-2619
5. Han G et al. Tenofovir alafenamide in blocking mother-to-child transmission of hepatitis B virus: A multi-center, prospective clinical study. ILC 2021;PO-797.
6. Segeral O et al. Immunoglobulin-free alternative strategy to prevent HBV mother to child transmission in Cambodia: preliminary results of the ANRS 12345 TA PROHM study. ILC 2021;OS-865
7. Lee D et al. Nucleos(t)ide analogue treatment is associated with lower risk of extrahepatic malignancy in chronic hepatitis B patients: A landmark study using nationwide claim data. LIC 2021; OS-691.
8. Jansen HLA et al. Switching from tenofovir disoproxil fumarate and/or other oral antivirals to tenofovir alafenamide in virally suppressed CHB patients with moderate or severe renal impairment or ESRD on HD: final week 96 efficacy and safety results from a phase 2 study. ILC 2021; PO-2395
9. Yuen MF et al. Repeat dosing of the GalNAc-siRNA AB-729 in subjects with chronic hepatitis B results in robust and sustained HBsAg suppression. ILC 2021; LBO-2764
10. Gane E et al. Safety and antiviral activity of VIR-2218, an X-targeting RNAi therapeutic, in participants with chronic hepatitis B infection: week 48 follow-up results. ILC 2021; OS-44
11. Yuen MF et al. Hepatitis B virus surface antigen inhibition with GSK3228836 (ISIS 505358) in chronic hepatitis B patients on stable nucleos(t)ide analogue regimen and in nucleos(t)ide analogue naïve patients: A phase 2a, randomized, double-blind, placebo-controlled study. ILC 2020; 73: S49-S50
12. You S et al. Treatment with GSK3228836 leads to HBsAg reduction and induction of interferon gamma related proteins and chemokines in a Phase 2a, randomized, double-blind, placebo-controlled study. ILC 2021; PO-2173.
13. Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol. 2010;7:31-40.
14. Assaleh T et al. Safety and efficacy of bulevirtide monotherapy and in combination with peginterferon alfa-2a in patients with chronic hepatitis delta: 24 weeks interim data of MYR204 phase 2b study. ILC 2021; PO-2717
15. Wedemeyer H et al. Bulevirtide monotherapy at low and high doses in patients with chronic hepatitis Delta: 24-week interim data of the phase 3 MYR301 study. ILC 2021; PO-2730
16. Wedemeyer H et al. Persistent long-term risk of liver related complications in hepatitis C virus patients after antiviral therapy - Data from the German Hepatitis C-Registry (DHC-R). ILC 2021; PO-52
17. D'Ambrosio R, et al. Incidence of liver and non-liver related events and mortality in a large cohort of HCV cirrhotics with an SVR to DAA: a 5-year single center study . ILC 2021; PO-697
18. EASL recommendations on treatment of hepatitis C: Final update of the series. Journal of Hepatology 2020;73:1170-1218
19. Degasperi E et al. Non-invasive tests to predict liver-related events in a single-center cohort of 486 HCV cirrhotic patients treated with DAA. ILC-2021; PO-717.
20.
https://www.who.int/news/item/25-06-2021-who-releases-first-ever-global-guidance-for-country-validation-of-viral-hepatitis-b-and-c-elimination
21. Tsertsvadze T et al. Progress towards achieving hepatitis C elimination in the country of Georgia, April 2015-December 2021. ILC-2021; PO-1553
22. Cox S et al. Achieving hepatitis C micro-elimination in prisons through high intensive test and treat (HITT) Interventions. ILC 2021; PO-637
22a. Increased serum ferritin levels predict long-term mortality in patients with NAFLD. ILC 2021. OS-635.
23. Nakajima A et al. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator (SPPARM alpha), improves liver stiffness by MRE and serum liver and lipid parameters in NAFLD: a randomized, double-blind, placebo-controlled phase 2 trial. ILC 2021; PO-681
24. Moreira de Macedo S, et al. The role of renin-angiotensin system modulation on treatment and prevention of liver diseases. Peptides. 2014;62:189-196.
25. Yokohama S, et al. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40:1222-5.
26. Zhang X et al. Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers are associated with lower risk of hepatocellular carcinoma and cirrhotic complications in patients with non-alcoholic fatty liver disease. ILC 2021;PO-931
27. Holmer M et al. Treatment of NAFLD with intermittent calorie restriction, low-carb high-fat diet or standard dietary advice: a randomized controlled trial. ILC 2021; PO-1627
28. Holmer M, et al. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet - a randomised controlled trial. JHEP Rep. 2021 Feb 17;3(3):100256.
29. Vilar-Gomez E., et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367-378.
30. Mallet V et al. Chronic liver disease and the risk of mortality after Covid-19: a national, retrospective, cohort study for 2020. ILC 2021; GS-1587
31. Mallet V, Beeker N, Bouam S, Sogni P, Pol S, for the Demosthenes researchgroup, Prognosis of French COVID-19 patients with chronic liver disease: a national retrospective cohort study for 2020, Journal of Hepatology 2021; in press, doi: https://doi.org/10.1016/j.jhep.2021.04.052.
32. Subhani M et al. Effect of COVID-19 on alcohol use disorder among hospitalised patients: a retrospective cohort control study. ILC 2021; OS-1381.
33. Hakimian D et al. Elderly with advanced liver fibrosis had lower response to Pfizer's SARS-CoV-2 vaccine response. ILC 2021; OS-2854
34. Aggeletopoulou I, et al. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017 Nov;27(6). doi: 10.1002/rmv.1942.
35. McCashland TM, et al. Pneumococcal vaccine response in cirrhosis and liver transplantation. J Infect Dis. 2000;181:757-60.
36. Harmala S, et al. Effectiveness of influenza vaccines in adults with chronic liver disease: a systematic review and meta-analysis. BMJ Open. 2019;9:e031070.
|
| |
|
 |
 |
|
|