| |
Tenofovir Alafenamide to Prevent Perinatal Hepatitis B Transmission: A Multicenter, Prospective, Observational Study
|
| |
| |
CID Jan 4 2021
Download the PDF here
Summary: Rare clinical data are available regarding TAF treatment during pregnancy. This multicenter, prospective, TDF-controlled, observational study demonstrated that TAF was safe for highly viremic pregnant women and their infants and reduced the mother-to-child transmission rate of HBV to 0%.
This study was accepted as an Oral Presentation (publication number: 0160) by the 2020 AASLD Annual Meeting and has been selected for inclusion in the Best of The Liver Meeting summary slide deck in the Hepatitis B category and awarded the AASLD International Early Career Investigator Award (to Dr. Qing-Lei Zeng).
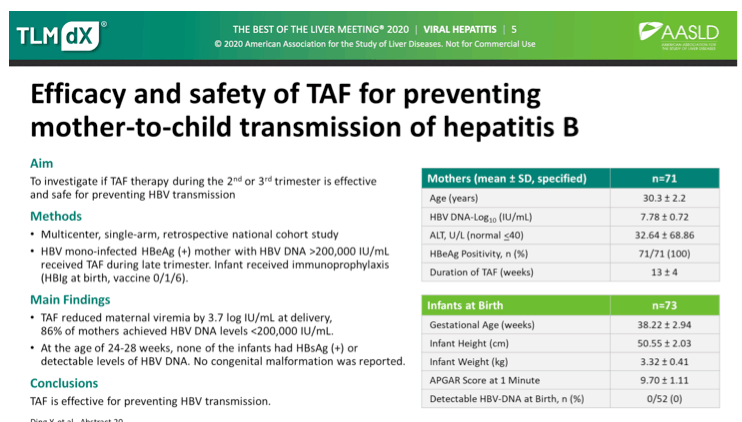
AASLD: Tenofovir alafenamide fumarate therapy for the prevention of hepatitis B vertical transmission in highly viremic mothers with chronic hepatitis B - (11/18/20)
AASLD: Tenofovir alafenamide to prevent perinatal hepatitis B transmission in mothers with high viral load: a multicenter, prospective, observational study. - (11/18/20)

In China, TAF was licensed for the treatment of CHB in December 2018; the drug label indicates, "TAF can be used during pregnancy if necessary", but it discourages breastfeeding when taking TAF. In real-world clinical practice, an increasing number of highly viremic pregnant Chinese women take TAF as antiviral prophylaxis for the prevention of MTCT of HBV. In this report, we describe the safety and effectiveness of TAF to prevent MTCT of HBV in a real-world setting.
Abstract
Background
Few safety and effectiveness results have been published regarding the administration of tenofovir alafenamide fumarate (TAF) during pregnancy for the prevention of mother-to-child transmission (MTCT) of hepatitis B virus (HBV).
Methods
In this multicenter prospective observational study, pregnant women with HBV DNA levels higher than 200,000 IU/ml who received TAF or tenofovir disoproxil fumarate (TDF) from gestational weeks 24-35 to delivery were 1:1 enrolled and followed until postpartum month 6. Infants received immunoprophylaxis. The primary endpoint was the safety of mothers and infants. The secondary endpoint was the hepatitis B surface antigen (HBsAg)-positive rate at 7 months for infants.
Results
In total, 116 and 116 mothers were enrolled, and 117 and 116 infants were born, in the TAF and TDF groups, respectively. TAF was well tolerated during a mean treatment duration of 11.0 weeks. The most common maternal adverse event was nausea (19.0%). One (0.9%), 3 (2.6%), and 9 (7.8%) mothers had abnormal alanine aminotransferase levels at delivery and at postpartum months 3 and 6, respectively. The TDF group had safety profiles that were comparable to those of the TAF group. No infants had birth defects in either group. The infants' physical and neurological development at birth and at 7 months in the TAF group were comparable with those in the TDF group. The HBsAg positive rate was 0% at 7 months in all 233 infants.
Conclusion
Antiviral prophylaxis with TAF was determined to be generally safe for both mothers and infants and reduced the MTCT rate to 0%.
RESULTS
Participants
A total of 1066 chronic HBV-infected pregnant women were assessed for eligibility from January 1 to July 31, 2019 (Fig. 1), and 232 mothers were eventually enrolled 1:1 for treatment with TAF or TDF. Among the 116 TAF-treated mothers with a mean age of 29.6 (± 4.5) years, 107 (92.2%) were HBeAg positive, and 9 (7.8%) were negative (Table 1). Mothers initiated TAF therapy at a mean gestational age of 28.2 (± 2.1) weeks; notably, 6 (5.2%) of them started between 33 and 35 gestational weeks. The TDF group had characteristics comparable to those of the TAF group at the timepoint of treatment initiation (Table 1). In total, 117 live newborns, including one pair of twins, were born in the TAF group, and 116 live newborns were born in the TDF group (Supplementary Table 1). Based on the mothers' personal preferences, 46 (39.3%) and 43 (37.1%) infants received breast milk in the TAF and TDF groups, respectively.
Safety of the Mothers
All 232 mothers completed the full course of TAF or TDF treatment, i.e., from baseline to delivery. In the TAF group, the mean treatment duration was 11.0 (± 2.5) weeks, and the shortest and longest treatment periods were 2 and 16 weeks, respectively; in the TDF group, the mean treatment duration was 11.1 (± 1.9) weeks, and the shortest and longest treatment periods were 5 and 16 weeks, respectively. TAF was well tolerated in the pregnant women, no mothers discontinued therapy because of adverse events (Table 2), and all discontinued therapy naturally at delivery. The most common perinatal adverse events and complications were nausea (19.0%) and premature rupture of membranes (12.9%). None of the mothers received amniocentesis during pregnancy. The TDF group had comparable adverse events and complications with the TAF group during treatment (Table 2).
In terms of laboratory abnormalities at delivery (Table 3), 35 (30.2%) mothers had anemia in the TAF group, and 14 (12.1%) of them were diagnosed with moderate anemia, which was defined as a hemoglobin concentration ranging from 70-99 g/L [14]. One (1.1%) and 3 (2.6%) mother(s) had an ALT higher than the ULN at delivery (51 U/L) and postpartum month 3 (47, 56, 60 U/L) in the TAF group, respectively. At postpartum month 6, 9 (7.8%) mothers had an ALT level higher than the ULN, with a mean level of 72.7 (± 18.0) U/L in the TAF group, a peak level of 100 U/L, and no mothers had ALT flares (in either group). Additionally, no significant differences were observed for serum creatinine levels between the timepoints of baseline, delivery, and postpartum months 3 and 6 (all P > 0.05) in the TAF group. The TDF group had laboratory abnormalities comparable to those of the TAF group at delivery and at postpartum months 3 and 6 (Table 3, no significant difference was found, P value data are not shown).
Safety of the Infants
In terms of infant safety, the mean gestational age of the 117 infants was 39.2 (± 1.4) weeks in the TAF group, 56 (47.9%) of them were born by cesarean section, and 4 (3.4%) of them were preterm infants (Supplementary Table 1). No infant had an Apgar score of less than 8 at 1 minute of birth in the TAF group. No congenital defects or malformations were observed in the TAF group. No severe abnormal signs or symptoms were observed among infants from birth to 7 months in the TAF group (Supplementary Table 2). The TDF group had characteristics and abnormal conditions comparable to those of the TAF group (Supplementary Tables 1 and 2).
The most common abnormal condition was prolonged (neonatal) jaundice in both groups [15], which was defined as neonatal jaundice lasting more than 2 weeks in term infants and more than 3 weeks in preterm infants, and it occurred in 15 (12.8%) and 13 (11.2%) infants in the TAF and TDF groups, respectively. These 28 infants with prolonged jaundice were either spontaneously resolved or cured by phototherapy alone before 2 months. The infants' physical and neurological development at birth and at 7 months in the TAF group were comparable to those in the TDF group or even slightly higher than the China national standards and WHO standards for children's growth (Table 4).
On-time Rate of Immunoprophylaxis
Among the 117 newborns in the TAF group, the time from birth to administration of the first doses of immunoprophylaxis was 6.6 (± 4.5) hours (Supplementary Table 1). Of the newborns, 89 (76.1%) received the first doses of HBIG and HBV vaccine within 12 hours of birth, and the remaining 28 (23.9%) received injections within 13-19 hours of birth because of the vaccination stations being closed at night (commonly from 18:00 to 08:00). For the second dose of HBV vaccine, 91 (77.8%) infants were injected at 1 month, 18 (15.4%) infants received injections 2-4 days after the one-month target because of personal reasons or that the vaccination stations were closed on weekends or holidays, and 8 (6.8%) were delayed for 2-4 weeks because of prolonged neonatal jaundice. With regard to the third dose of HBV vaccine, 101 (86.3%) infants were injected at 6 months, and 16 (13.7%) were delayed for 1-4 weeks because of personal reasons or traffic influenced by the coronavirus disease 2019 in Henan Province, China [16]. The TDF group had comparable on-time rates of immunoprophylaxis with the TAF group (Supplementary Table 1).
Effectiveness in the Mothers and Infants
The mean decreases in serum HBV DNA levels in TAF- and TDF-treated mothers were 4.3 (± 0.6) log10 IU/ml and 4.4 (± 0.7) log10 IU/ml, leading to mean viral loads of 3.5 (± 0.9) log10 IU/ml and 3.4 (± 1.0) log10 IU/ml at delivery, respectively (Table 3). Upon delivery, 100% (232/232) of mothers achieved HBV DNA levels < 200,000 IU/ml in both groups. No mother had HBeAg or HBsAg clearance at delivery or at postpartum months 3 and 6 in either group. At the 7-month visit for infants, no infant was positive for HBsAg in either group: the MTCT rate was 0%. Additionally, 115 (98.3%) and 115 (99.1%) infants had positive anti-HBs, with mean anti-HBs levels of 444.4 (± 302.9) mIU/ml and 447.7 (± 277.8) mIU/ml, and 41 (35.0%) and 39 (33.6%) infants had positive anti-HBc at 7 months in the TAF and TDF groups, respectively.
|
|
| |
| |
|
|
|