| |
HCV SCREENING AND LINKAGE TO CARE AT COMMUNITY HOSPITAL EMERGENCY DEPARTMENT: RETROSPECTIVE REVIEW OF PATIENT CHARACTERISTICS AND FUTURE IMPLICATIONS
|
| |
| |
We are not on a path to HCV elimination here in the USA or globally with several key presentations at AASLD reporting that we must implement real universal routine HCV screening & linkage to care of 80% to make headway & reduce deaths, otherwise we are many many years away. We have not made much progress towards elimination the USA over the past few years since the 2nd & 3rd generation DAAs have become available. There has been very modest incremental small steps forward like eliminating some restrictions, expanding treatment, additional screening, but in the big picture this progress has NOT put on us on a real path to HCV elimination as evidenced by these data reported at AASLD. Unfortunately COVID has set us back too. One study at AASLD predicted an 18 month delay innHCV screening etc. It appears that access to clean syringes is impacted by COVID, so is HCV screening with many screening projects shut down & many afraid to get screened or visit clinics, even before COVID it was estimated 40% in the USA remain HCV undiagnosed. Below are several AASLD presentations I chose to highlight here that reflect important treatment & screening information & advances. Some screening programs presented at AASLD discuss how they have tried with some success to screen & treat during COVID. PWUD still are not adequately accessing treatment, young women of child bearing age are not adequately screened & accessing treatment, pregnant women are not screened enough. The homeless & prisoners do not get screened & treated adequately. However just below you will see improved liver stiffness as measured by FibroScan does reflect improved outcomes. We have known this for years but just below is study that HCV recurrence after SVR is close to zero if not zero, except of course due to reinfection. Novel screening programs are highlighted below. NYS, Louisianna & perhaps Wash State have made some commitment. Perhaps NYS has made the biggest financial commitment with $5 mill allocated per year for several years started a few years ago, but that is botenough to call it a real HCV elimination program. Louisianna is as I understand it making some commitment & so has Wash State as these are the 2 states that cut deals with Abbvie & Gilead on pricing for DAAs, but the actual financial commitments by the states remain uncertain.
AASLD: GLOBAL PREVALENCE OF HEPATITIS C VIRUS IN WOMEN OF CHILDBEARING AGE IN 2019: A MODELING STUDY - (12/21/20)
HBV & HCV & Accelerated Senescence: premature aging - (12/18/20)
Hepatitis A, B and C in New York City: 2019 Annual Report - (12/16/20)
New York State Department of Health Hepatitis B and C Annual Report 2018 - (12/18/20)
Universal Screening Real Implementation is Required for HCV Elimination & Reduced Mortality
AASLD: All Adult HCV Testing Global and US Implementation - (11/16/20) John Ward
AASLD: Determining Feasibility of Hepatitis C Elimination in the United States Using a Simulation Model - (11/16/20)
AASLD: Country and WHO Regional Trends for Hepatitis C Virus (HCV) Mortality, 1990-2019: An Analysis of the Global Burden of Disease (GBD) Study - (11/17/20)
AASLD: Liver stiffness regression after sustained virological response by direct-acting antivirals reduces the risk of outcomes - (11/13/20)
AASLD: The risk of HCV recurrence in HCV infected patients treated with DAA after achieving a sustained virological response: a comprehensive analysis - (11/13/20)
AASLD: AUTOMATED VIRAL HEPATITIS (HCV AND HBV) SCREENING: LESSONS ON EXPANSION & SUSTAINABILITY AMIDST THE COVID-19 PANDEMIC - (12/21/20) NJ
AASLD: ELIMINATING HEPATITIS C: A COMPREHENSIVE PROGRAM OF DIGITAL CASE FINDING AND LINKAGE TO CARE ACROSS A LARGE URBAN HEALTHCARE SYSTEM - (11/13/20) NYC
AASLD: Early phase implementation of routine opt out hepatitis C testing in the Philadelphia jail system - (11/28/20) Philadelphia
AASLD: Provision of Hepatitis C Care in a Federally Qualified Health Center during the COVID-19 Pandemic - (11/13/20) Philadelphia
AASLD: A hospital free of Hepatitis C: Hepatitis C virus screening program in an Emergency Department of a tertiary hospital of a high-income country. (Preliminary results) - (11/16/20)
AASLD: Treatment As Prevention: Addressing HCV in Persons Who Inject Drugs (PWID) - (11/28/20)
AASLD: POINT-OF-CARE HEPATITIS C TESTING AND TREATING STRATEGY IN PEOPLE WHO INJECT DRUGS IN HARM REDUCTION AND ADDICTION CENTERS FOR HEPATITIS C ELIMINATION - (11/19/20)
AASLD: Active Drug Use Is Not Associated with Treatment Failure Among PWID Patients With Variable Adherence to HCV Treatment - (11/16/20)
AASLD: Variable Adherence To HCV Treatment Among People Who Inject Drugs Treated with 8 Versus 12 Weeks of Antiviral Therapy Resulted In High Rates of SVR - (11/16/20)
AASLD: Real-World Outcomes in Patients With Chronic Hepatitis C Virus Infection and Substance Use Disorders Treated With Glecaprevir/Pibrentasvir for 8 Weeks: A Pooled Analysis of Multinational Postmarketing Observational Studies - (11/19/20)
AASLD: HCV elimination in homeless patients is possible: a pooled real-world analysis of homeless patients with HCV treated with sofosbuvir/velpatasvir (SOF/VEL) for 12 weeks - (11/13/20)
AASLD: The value of sofosbuvir/velpatasvir (SOF/VEL) as a pangenotypic and panfibrotic HCV treatment in implementing a test-and-treat strategy in prisons: real-world care management from 6 countries - (11/13/20)
AASLD: A Multisite Randomized Pragmatic Trial of Patient-Centered Models of Hepatitis C Treatment for People Who Inject Drugs: The HERO Study - Hepatitis C Real Options - (11/16/20)
AASLD: The "Keep It Simple and Safe" Approach to HCV Treatment: Primary Outcomes from the ACTG A5360 (MINMON) Study - (11/19/20)
AASLD: Low adherence to infant HCV testing guidelines among pregnant women with HCV cirrhosis - (11/16/20)
AASLD: COST-EFFECTIVENESS OF ANTENATAL RESCREENING AMONG PREGNANT WOMEN FOR HEPATITIS C IN THE UNITED STATES - (11/20/20)
AASLD: State Policies Limiting Progress Towards HCV Elimination in the U.S. - (11/20/20)
AASLD: Effectiveness of sofosbuvir/velpatasvir (SOF/VEL) in patients with chronic HCV infection and mental health disorders: real-world care management from 8 countries - (11/13/20)
----------------------------------------------
HCV SCREENING AND LINKAGE TO CARE AT COMMUNITY HOSPITAL EMERGENCY DEPARTMENT: RETROSPECTIVE REVIEW OF PATIENT CHARACTERISTICS AND FUTURE IMPLICATIONS
AASLD 2020 Nov 11-16
Ji Seok Park1, Hillary Cohen2 and Judy Wong2, (1)Department of Gastroenterology, Hepatology and Clinical Nutrition, Cleveland Clinic, (2)Department of Emergency Medicine, Englewood Health
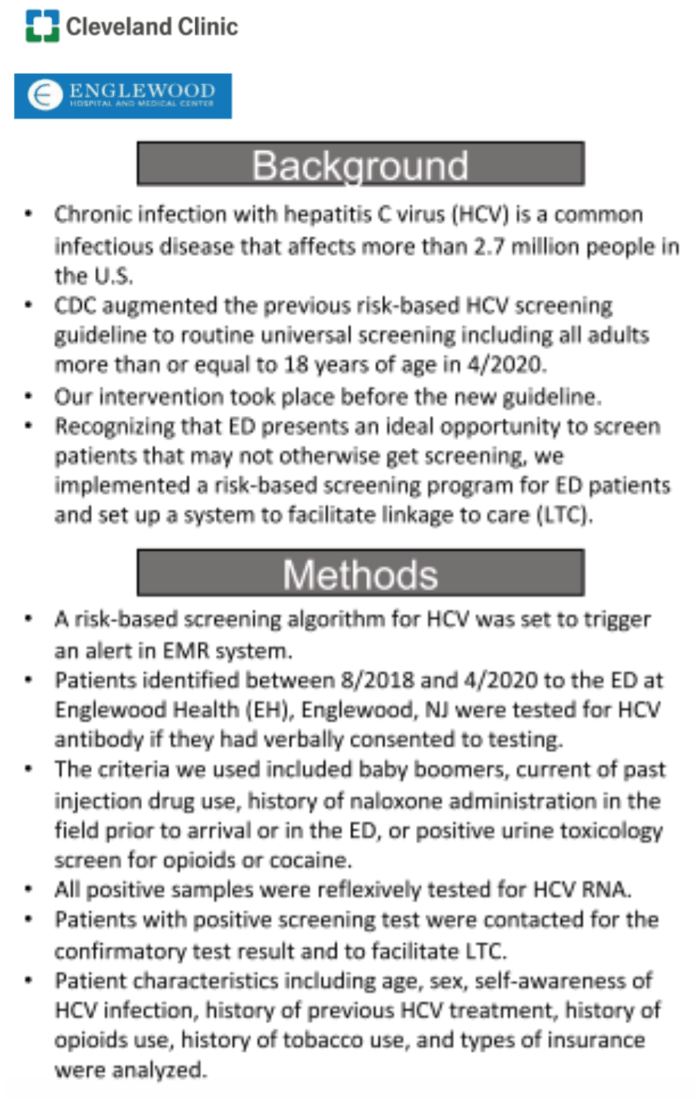
Background: Chronic infection with hepatitis C virus (HCV) is a common infectious disease that affects more than 2 .7 million people in the U .S. Due to high mortality and morbidity associated with chronic hepatitis C, CDC augmented the previous risk- based HCV screening guideline to routine universal screening including all adults more than or equal to 18 years of age. Our intervention took place before the new guideline. Recognizing that emergency department (ED) presents an ideal opportunity to screen patients that may not otherwise get screening, we implemented a risk-based screening program for ED patients and set up a system to facilitate linkage to care (LTC) .
Methods: A risk-based screening algorithm for HCV was set to trigger an alert in Epic electronic medical record (EMR) system. Patients identified between Aug 2018 and Apr 2020 to the ED at Englewood Health (EH), Englewood, NJ were tested for HCV antibody if they had verbally consented to testing. The criteria we used included baby boomers, current of past injection drug use, history of naloxone administration in the field prior to arrival or in the ED, or positive urine toxicology screen for opioids or cocaine . All positive samples were reflexively tested for HCV RNA. Patients with positive screening test were contacted for the confirmatory test result and to facilitate LTC . Patient characteristics including age, sex, self-awareness of HCV infection, history of previous HCV treatment, history of opioids use, history of tobacco use, and types of insurance were analyzed.
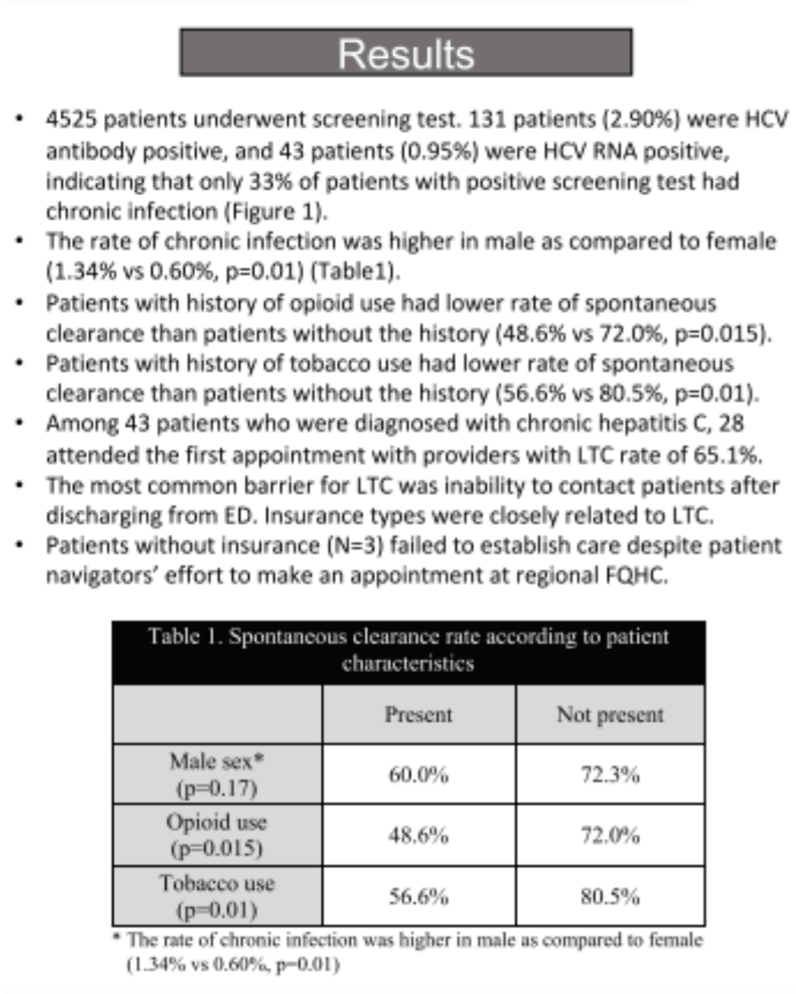
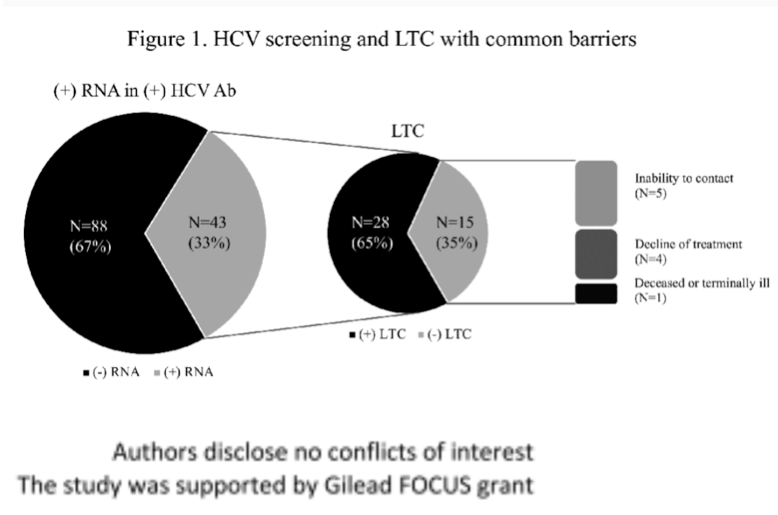
Results: 4525 patients underwent screening test. 131 patients (2.90%) were HCV antibody positive, and 43 patients (0.95%) were HCV RNA positive, indicating that only 33% of patients with positive screening test had chronic infection. The rate of chronic infection was higher in male as compared to female (1 .34% vs 0 .60%, p=0 .01). Patients with history of opioid use had lower rate of spontaneous clearance than patients without the history (48 .6% vs 72 .0%, p=0 .015. Patients with history of tobacco use had lower rate of spontaneous clearance than patients without the history (56.6% vs 80.5%, p=0 .01). Among 43 patients who were diagnosed with chronic hepatitis C, 28 attended the first appointment with providers with LTC rate of 65.1% (Figure 1) . The most common barrier for LTC was inability to contact patients after discharging from ED. Insurance types were closely related to LTC.
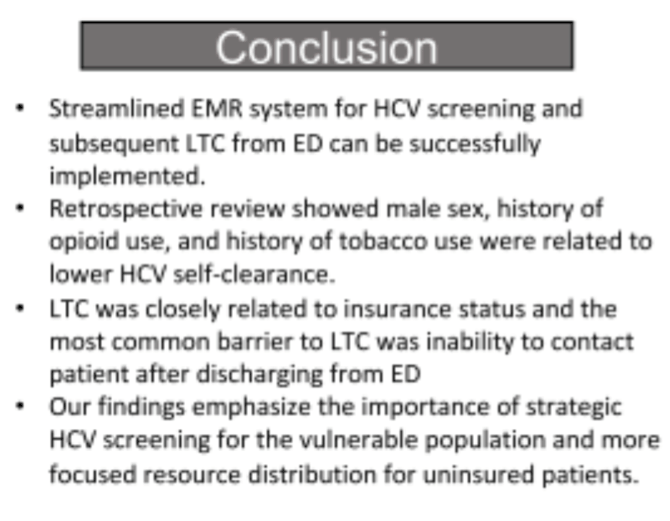
Conclusion: Streamlined EMR system for HCV screening and subsequent LTC from ED can be successfully implemented . Retrospective review showed male sex, history of opioid use, and history of tobacco use were related to lower HCV self-clearance. Furthermore, LTC was closely related to insurance status and the most common barrier was inability to contact patient after discharging from ED. These findings emphasize the importance of strategic HCV screening for the vulnerable population and more focused resource distribution for uninsured patients.

| |
| |
| |
|
|
|