| |
CVD Score Predicts Frailty? - Cardiovascular risk score associations
with frailty in men and women with or at risk for HIV
|
| |
| |
Download the PDF here
AIDS Feb 1 2022 Kuniholm, Mark H.a; Vásquez, Elizabetha; Appleton, Allison A.a; Kingsley, Lawrenceb; Palella, Frank J.c; Budoff, Matthewd; Michos, Erin D.e; Fox, Erving; Jones, Deborahh; Adimora, Adaora A.i; Ofotokun, Ighoj; D'souza, Gypsyamberf; Weber, Kathleen M.k; Tien, Phyllis C.l; Plankey, Michaelm; Sharma, Anjalin; Gustafson, Deborah R.o
Associations of individual CVD risk factors including hypertension, diabetes, smoking, and abdominal obesity have been observed with prevalent and/or incident frailty in middle-aged and older PWH and seronegative adults [3,16,34,40-42]. Few studies, however, have considered CVD risk scores as used in clinical practice in relation to frailty [43]. We observed that high 10-year CVD risk as defined by 2013 ACC/AHA guidelines was positively associated with frailty among both men and women regardless of HIV serostatus. In contrast, high CHD risk as defined by 2001 ATP-III guidelines was positively associated with frailty among men only. These findings are important as frailty is common in middle-aged populations of PWH like MACS and WIHS in the United States, and among some middle-aged PWH living in low-income and middle-income countries [7].
In conclusion, these data suggest that CVD risk prediction tools may be valuable in clinical practice to identify PWH and seronegative adults who may benefit from frailty phenotyping to reduce consequences of frailty onset including falls, hospitalization, disability, and mortality. Unfortunately, one of the reasons that frailty is not assessed more often clinically is lack of consensus around a single reliable metric. Frailty screening under accumulation of deficit models may be well suited to screening patients in ICUs [55]. For example, 234 568 critically ill patients were screened under a single protocol [56]. Protocols for physical frailty assessment have included hospitalized patients as well as community-dwelling participants, those living in long-term care/assisted living facilities and out-patients [57]. It may be that optimal frailty screening strategies will differ according to the population to be screened. Ultimately implementation science strategies are needed to assess if, how and when CVD risk assessment should be part of best practices for frailty identification and intervention in diverse settings among men and women with or at risk for HIV.
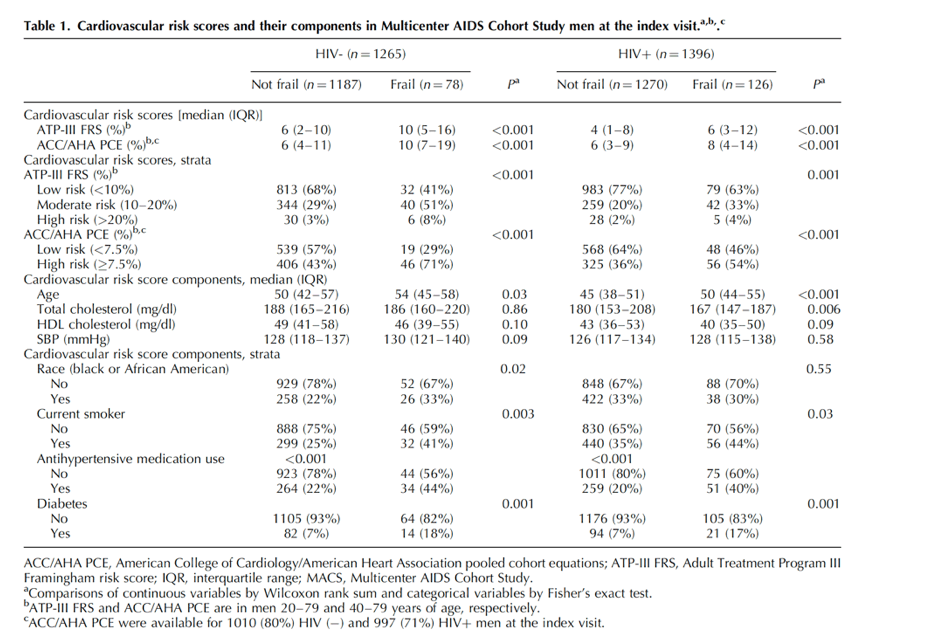
CVD risk score components associated with frailty in both seronegative men and MWH included age, smoking, antihypertensive medication use, and diabetes (all P < 0.05). In addition, among seronegative men, those who were frail were more likely to be black or African American, whereas frail MWH had lower total cholesterol (P = 0.006).
Some components of ATP-III FRS and ACC/AHA PCE differed by frailty status at the index visit. Women who were frail were older, more likely to smoke, use antihypertensive medication and be diabetic than women who were not frail, regardless of HIV serostatus (all P < 0.05); no differences were observed by frailty status in total and HDL cholesterol. SBP was higher among frail vs. nonfrail seronegative women (P = 0.03) but not in WWH (P = 0.28).
Frailty is characterized by physical weakness and a marked decline of physiologic reserve [1]. Higher prevalence of frailty has been reported among people with HIV (PWH) compared with adults without HIV infection in the United States [2-4], Europe [5], China [6], and Africa [7], suggesting that PWH worldwide are at increased risk for frailty. Risk factors common to cardiovascular disease (CVD) and frailty, such as older age, presence of comorbidities, smoking, physical inactivity, and poor nutrition exist, yet the relationship between CVD and frailty in PWH is not well established [8,9]. Cross-sectional research in the Multicenter AIDS Cohort Study (MACS) demonstrated that the Fried Frailty Phenotype (FFP) was associated with subclinical coronary atherosclerosis among HIV seronegative (SN) men but not among men living with HIV (MWH) [10]. Cross-sectional data in the Women's Interagency HIV Study (WIHS) showed that hypertension and cigarette smoking were associated with the FFP during middle age [3]. Some groups have suggested that frailty reflects the interaction between multiple morbidities and disability, and hence CVD contributes to frailty [11]. It may also be argued that frailty is a biologic syndrome distinct from either comorbidities or disability. Notably, in the Cardiovascular Health Study, 7% of those who were frail according to FFP criteria had no chronic diseases [1]. Among persons without HIV, prevalence of frailty - defined by the FFP, the Frailty Index [12] or other frailty measures - was greater in those with vs. without CVD [13-16]. Moreover, among persons without CVD, risk factors for CVD were associated with frailty [15,16].
CVD risk factors are used in clinical practice as part of CVD event risk prediction and to guide therapeutic decision making in adults, including PWH [17-23]. However, traditional CVD risk prediction tools underperform among PWH, and differ by sociodemographic and race/ethnicity factors [18,19,22]. The 2001 National Cholesterol Education Program Adult Treatment Program III (ATP-III) [24] and the 2013 American College of Cardiology/American Heart Association (ACC/AHA) [25] guidelines, with corresponding risk scores, have been widely used for primary prevention of CVD. These risk scores have important differences; however, as they were released a decade apart, there was an expansion of statin eligibility in the 2013 ACC/AHA guidelines compared with the 2001 ATP-III [26]. The ATP-III recommendations for primary prevention are based on the Framingham risk score (FRS) for coronary heart disease (CHD). The ATP-III FRS includes the components: sex, age, total cholesterol, high-density lipoprotein (HDL) cholesterol, smoking status, SBP and antihypertensive medication use [24]. The ACC/AHA recommendations are based on pooled cohort equations (PCE) for atherosclerotic cardiovascular disease (ASCVD) with components of race (white/African American) and diabetes, in addition to the components included in ATP-III FRS [25].
Our objective was to consider ATP-III FRS and ACC/AHA PCE risk scores in association with frailty, defined using the FFP, among men and womenenrolled in the MACS and WIHS. Understanding these associations could be important as the FFP requires assessment of physical performance (grip strength and walking speed) and may be ascertained less often than CVD risk in clinical practice. Notably, this article is among the first to consider CVD risk profiles as a predictor of frailty among two large longitudinal cohorts of men and women living with or at risk for HIV who have been followed at 14 clinical sites across the United States.
Study populations
The MACS and WIHS (now known as the MACS/WIHS Combined Cohort Study [27]) were prospective multicenter cohorts of United States men and women living with or without HIV. Eligibility criteria, study protocols, and follow-up procedures for the MACS and WIHS have been previously described [28-32]. Briefly, MACS participants were recruited at 4 sites across 3 time periods starting in 1984, and WIHS participants were recruited at 10 sites over four time periods starting in 1994. Data for both cohorts were collected using structured in-person interviews and standardized physical and laboratory assessments, with study visits occurring every 6 months. As part of a detailed medication history, participants self-reported all prescribed medications consumed, even transiently, since their last study visit. Institutional review boards at the respective clinical research centers approved the MACS and WIHS study protocols, and all participants provided written informed consent.
Predictor ascertainment: cardiovascular risk score calculations
For each participant, we calculated ATP-III FRS [24] and ACC/AHA PCE [25] cardiovascular risk scores using: age, race (black or African American vs. other race), hypertension treatment and smoking status (yes/no); and clinically measured SBP (mmHg), diabetes (fasting glucose ≥126 mg/dl or taking diabetes medication at the visit), and total and HDL cholesterol measured on a Roche Modular automated system (Roche Diagnostics Corporation, Indianapolis, Indiana, USA). For ATP-III FRS, 10-year risk of CHD was defined as high (>20%), moderate (10-20%), or low (<10%) [24]. Persons with ACC/AHA PCE at least 7.5% were considered at high 10-year risk of ASCVD whereas those with ACC/AHA PCE less than 7.5% were at low 10-year risk of ASCVD [25]. These definitions of risk are consistent with each respective guideline.
Discussion
Associations of individual CVD risk factors including hypertension, diabetes, smoking, and abdominal obesity have been observed with prevalent and/or incident frailty in middle-aged and older PWH and seronegative adults [3,16,34,40-42]. Few studies, however, have considered CVD risk scores as used in clinical practice in relation to frailty [43]. We observed that high 10-year CVD risk as defined by 2013 ACC/AHA guidelines was positively associated with frailty among both men and women regardless of HIV serostatus. In contrast, high CHD risk as defined by 2001 ATP-III guidelines was positively associated with frailty among men only. These findings are important as frailty is common in middle-aged populations of PWH like MACS and WIHS in the United States, and among some middle-aged PWH living in low-income and middle-income countries [7].
ATP-III FRS risk scores were low (median: 1- 2%) at the index visit among women regardless of HIV serotatus. Age is included not only as a component of the ATP-III FRS risk score equations but also serves as a modifier of the contribution of smoking and total cholesterol [24]. It is possible that the relatively young age of WIHS women with and without frailty (median ages: 42-48 years) contributed to these low FRS risk scores. Age is included differently in ACC/AHA PCE risk score calculations [25], as PCE predicts both CHD and stroke. This could explain why ACC/AHA PCE risk scores were higher (median: 3-5%) than those of ATP-III FRS at the WIHS index visit. However, in a sample constituted of predominantly male Dutch PWH, ATP-III FRS risk scores were higher than ACC/AHA PCE risk scores [18], whereas among MACS seronegative men, the two algorithms yielded similar CVD risk. Another difference between ATP-III FRS and ACC/AHA PCE is inclusion of African American race in ACC/AHA PCE equations - WIHS women are mostly African American whereas MACS includes majority white men.
Associations of ATP-III FRS and ACC/AHA PCE risk scores with frailty were stronger among the seronegative men as compared with MWH, similar to data from a MACS study in which FFP was associated with subclinical coronary atherosclerosis among seronegative men but not MWH [10]. One hypothesis to explain these observations is that a greater proportion of the variance in frailty is explained by CVD in seronegative men. HIV infection, including in MWH who consume ART with 100% adherence, may be associated with immune activation and systemic inflammation [44,45], which may also contribute to frailty, but to a lesser extent than CVD. Regardless, our findings are consistent with the concept that CVD contributes to frailty [11]. For example, among participants in the AIDS Clinical Trials Group A5322 HIV Infection, Aging, Immune Function Long-Term Observational Study, frailty at baseline was associated with incident CVD with incidence rate ratio of 3.83 (95% CI: 1.59-9.23) over a median of 4.0 years of follow-up [46].
This study has a number of strengths including clinically standardized frailty measurements and CVD risk factors, every 6-month follow-up, multisite representation across the United States, and inclusion of control groups of both women and men living without HIV. These strengths notwithstanding, limitations should be considered in interpretating our findings. First, there is no consensus on a single definition of frailty. The FFP reflects a physical phenotype whereas other measures are based on accumulation of deficits or other frameworks [47,48]. Moreover, optimal cut-points and algorithms to define ‘weakness’ (e.g. grip/BMI, grip/body weight) and ‘slowness’ (e.g. walking speed/body height) in relation to the frailty outcome are an area of active investigation [49,50]. Different methods to define the FFP components might lead to different findings as compared with FFP based on average grip and walk speed and comparisons by HIV serostatus, as in this investigation and prior studies [3,10,34]. Second, although ATP-III FRS and 2013 ACC/AHA PCE have been widely used in clinical practice, they represent two of several validated CVD risk scores (e.g. FRS ASCVD [51], 2019 ACC/AHA PCE [52], Systematic Coronary Risk Evaluation(SCORE) [53], and Data collection on Adverse Effects of Anti-HIV Drugs Study (D:A:D) [54]). Different CVD risk equations may have different associations with frailty as for CVD events [22,23] and subclinical CVD [20], which can vary by race/ethnicity [19]. Notably, the 2019 ACC/AHA guideline uses the same PCE as the 2013 ACC/AHA guideline. However, at least 7.5% 10-year ASCVD risk is considered high risk in the 2013 guideline but at least 7.5 to less than 20% is considered intermediate risk in the 2019 guideline, and HIV infection is considered a risk-enhancing factor that would favor initiation of statin therapy particularly in borderline (5 to <7.5%) and intermediate-risk groups [52]. Finally, our study of frailty did not exclude participants who might have been excluded in a study of cardiovascular endpoints - those with self-reported CVD (myocardial infarction or heart attack, revascularization or angioplasty, transient ischemic attack/stroke, angina or hospitalization for heart condition).
In conclusion, these data suggest that CVD risk prediction tools may be valuable in clinical practice to identify PWH and seronegative adults who may benefit from frailty phenotyping to reduce consequences of frailty onset including falls, hospitalization, disability, and mortality. Unfortunately, one of the reasons that frailty is not assessed more often clinically is lack of consensus around a single reliable metric. Frailty screening under accumulation of deficit models may be well suited to screening patients in ICUs [55]. For example, 234 568 critically ill patients were screened under a single protocol [56]. Protocols for physical frailty assessment have included hospitalized patients as well as community-dwelling participants, those living in long-term care/assisted living facilities and out-patients [57]. It may be that optimal frailty screening strategies will differ according to the population to be screened. Ultimately implementation science strategies are needed to assess if, how and when CVD risk assessment should be part of best practices for frailty identification and intervention in diverse settings among men and women with or at risk for HIV.
|
|
| |
| |
|
|
|