| |
Dolutegravir as First- or Second-Line Treatment for HIV-1 Infection in Children
|
| |
| |
Download the PDF here
NEJM Dec 30 2021 A. Turkova, E. White, H.A. Mujuru, A.R. Kekitiinwa, C.M. Kityo, A. Violari, A. Lugemwa, T.R. Cressey, P. Musoke, E. Variava, M.F. Cotton, M. Archary, T. Puthanakit, O. Behuhuma, R. Kobbe, S.B. Welch, M. Bwakura Dangarembizi, P. Amuge, E. Kaudha, L. Barlow Mosha, S. Makumbi, N. Ramsagar, C. Ngampiyaskul, G. Musoro, L. Atwine, A. Liberty, V. Musiime, D. Bbuye, G.M. Ahimbisibwe, S. Chalermpantmetagul, S. Ali, T. Sarfati, B. Wynne, C. Shakeshaft, A. Colbers, N. Klein, S. Bernays, Y. Saïdi, A. Coelho, T. Grossele, A. Compagnucci, C. Giaquinto, P. Rojo, D. Ford, and D.M. Gibb, for the ODYSSEY Trial Team*
Abstract
Background
Children with human immunodeficiency virus type 1 (HIV-1) infection have limited options for effective antiretroviral treatment (ART).
Methods
We conducted an open-label, randomized, noninferiority trial comparing three-drug ART based on the HIV integrase inhibitor dolutegravir with standard care (non-dolutegravir-based ART) in children and adolescents starting first- or second-line ART. The primary end point was the proportion of participants with virologic or clinical treatment failure by 96 weeks, as estimated by the Kaplan-Meier method. Safety was assessed.
Results
From September 2016 through June 2018, a total of 707 children and adolescents who weighed at least 14 kg were randomly assigned to receive dolutegravir-based ART (350 participants) or standard care (357). The median age was 12.2 years (range, 2.9 to 18.0), the median weight was 30.7 kg (range, 14.0 to 85.0), and 49% of the participants were girls. By design, 311 participants (44%) started first-line ART (with 92% of those in the standard-care group receiving efavirenz-based ART), and 396 (56%) started second-line ART (with 98% of those in the standard-care group receiving boosted protease inhibitor-based ART). The median follow-up was 142 weeks.
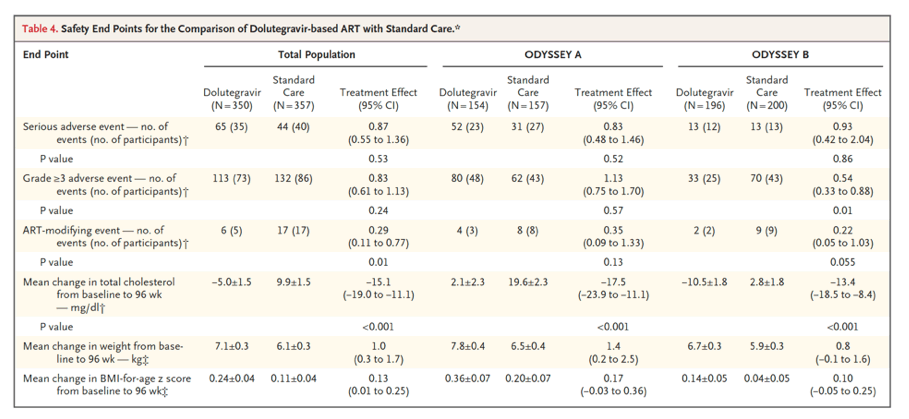
By 96 weeks, 47 participants in the dolutegravir group and 75 in the standard-care group had treatment failure (estimated probability, 0.14 vs. 0.22; difference, -0.08; 95% confidence interval, -0.14 to -0.03; P=0.004). Treatment effects were similar with first- and second-line therapies (P=0.16 for heterogeneity). A total of 35 participants in the dolutegravir group and 40 in the standard-care group had at least one serious adverse event (P=0.53), and 73 and 86, respectively, had at least one adverse event of grade 3 or higher (P=0.24). At least one ART-modifying adverse event occurred in 5 participants in the dolutegravir group and in 17 in the standard-care group (P=0.01).
Conclusions
In this trial involving children and adolescents with HIV-1 infection who were starting first- or second-line treatment, dolutegravir-based ART was superior to standard care.
(Funded by ViiV Healthcare; ODYSSEY ClinicalTrials.gov number, NCT02259127. opens in new tab; EUDRACT number, 2014-002632-14. opens in new tab; and ISRCTN number, ISRCTN91737921. opens in new tab.)
The randomized ODYSSEY trial showed evidence of the superior efficacy of dolutegravir-based ART, as compared with standard care, in children and adolescents starting first-line and second-line ART. The risk of treatment failure was approximately 40% lower with dolutegravir-based ART than with standard care. Superior efficacy was evident by 48 weeks, was sustained to 144 weeks, and was consistent across age, weight bands, and NRTI backbone therapies. No antiviral resistance was observed over a period of approximately 2 years in children and adolescents who started dolutegravir-based first-line ART, which suggests a higher barrier to INSTI resistance and protection against NRTI resistance than with mainly NNRTI-based first-line standard care. The occurrence of new INSTI resistance in 4 participants who received dolutegravir-based second-line ART highlights the need for ongoing adherence support among children and adolescents starting second-line treatment. Retention in the trial was excellent, and the incidence of treatment failure was low despite infrequent real-time viral-load monitoring in most participants and no requirement for resistance testing to guide the choice of NRTI backbone therapy for second-line treatment. Results are therefore generalizable across various settings, particularly in Africa, where most children with HIV-1 infection live.
Efficacy
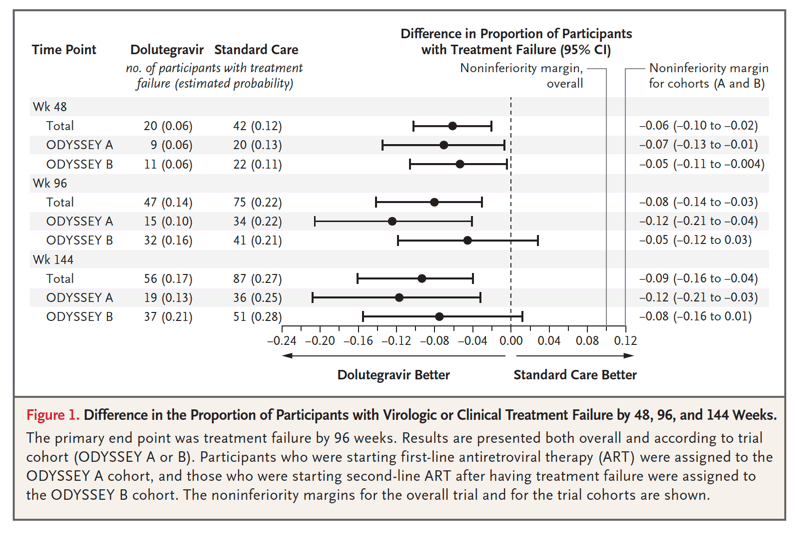
A total of 47 participants had protocol-defined treatment failure by 96 weeks (estimated probability of treatment failure, 0.14) in the dolutegravir group, as compared with 75 participants (estimated probability, 0.22) in the standard-care group (difference, -0.08; 95% confidence interval [CI], -0.14 to -0.03; P=0.004) (Figure 1). Results were similar in the per-protocol analysis (difference in estimated probability, -0.07; 95% CI, -0.12 to -0.01) and in sensitivity analyses (Figs. S7.2 through S7.7). Among participants who met the primary end point, 40 in the dolutegravir group and 67 in the standard-care group had virologic treatment failure. Seven participants in the dolutegravir group and 8 in the standard-care group were categorized as having had treatment failure on the basis of a new or recurrent WHO stage 4 or severe WHO stage 3 event or death (Table 2). Treatment effects were similar in the ODYSSEY A and B cohorts (P=0.16 for heterogeneity) (Figure 1).
In the overall trial population, the adjusted hazard ratio for treatment failure at 96 weeks was 0.60 (95% CI, 0.42 to 0.86). In prespecified exploratory analyses, there was no evidence that the treatment effect differed according to sex; baseline age, weight, CD4 lymphocyte percentage (CD4 percentage), and viral load; randomization stratification factors (choice of NRTI backbone therapy and availability of resistance testing); or calendar time (indicator of changes in the recommended dolutegravir doses and formulations). Adjustment for baseline viral load (for which there was by chance a slight baseline imbalance between the treatment groups) did not affect results (Fig. S7.8). The difference between treatment groups was present by week 48 and sustained to week 144 (Figure 1).
At weeks 48 and 96, the percentages of participants with a viral load of less than 400 copies per milliliter and of less than 50 copies per milliliter were similar in the two treatment groups, both overall and in the ODYSSEY A and B cohorts. Assessment of these cross-sectional viral loads did not include consideration of previous viral rebound (in which a child or adolescent had resuppression with the same regimen or after treatment change) (Table 2). There were 10 new or recurrent WHO stage 4 or severe WHO stage 3 events or deaths (in 8 participants) in the dolutegravir group and 8 such events (in 8 participants) in the standard-care group, with no evidence of differences between groups across the total follow-up or by 96 weeks (Tables S7.18 and S7.19). At 96 weeks, the CD4 count did not differ significantly between the groups (Table 2), but we observed some evidence of a difference in the increase in the CD4 count from baseline in favor of the dolutegravir group over the standard-care group (adjusted mean difference through 96 weeks, 27 cells per cubic millimeter; 95% CI, 0 to 53); the results were similar for the CD4 percentage (Table S8.4).
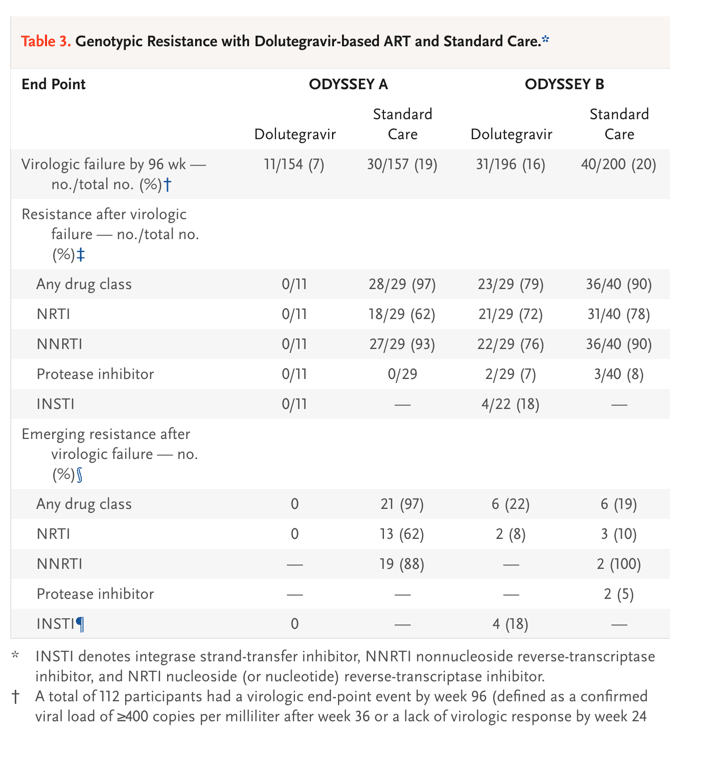
Among participants receiving first-line therapy (ODYSSEY A cohort), none of those in the dolutegravir group had a major drug-resistance mutation (as defined by the International AIDS Society) after treatment failure. Of 29 participants in the standard-care group who had virologic treatment failure by week 96 and had a post-treatment failure resistance test available for the drug class, 18 participants (62%) had NRTI-related mutations, 27 (93%) had NNRTI-related mutations, and none had protease inhibitor-related mutations; most of the mutations were new (Table 3).
Among participants receiving second-line therapy (ODYSSEY B cohort), 23 of 29 (79%) in the dolutegravir group and 36 of 40 (90%) in the standard-care group had at least one major mutation after treatment failure. Among participants who had exposure to the drug class, an INSTI-related mutation developed in 4 participants in the dolutegravir group (of whom 3 were receiving twice-daily zidovudine and lamivudine) and 2 had new NRTI mutations; in the standard-care group, 3 had new NRTI-related mutations, 2 participants had new NNRTI-related mutations, and 2 had new protease inhibitor-related mutations (Table 3 and S7.21).
|
|
| |
| |
|
|
|