| |
Weight and Metabolic Changes After Switching From Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in People Living With HIV - A Cohort Study
|
| |
| |
Download the PDF here
16 March 2021
Annals of Internal Medicine - Bernard Surial, MD*; Catrina Mugglin, MD, MSc*; Alexandra Calmy, MD, PhD; Matthias Cavassini, MD;Huldrych F. Günthard, MD; Marcel Stöckle, MD; Enos Bernasconi, MD; Patrick Schmid, MD; Philip E. Tarr, MD;Hansjakob Furrer, MD; Bruno Ledergerber, PhD; Gilles Wandeler, MD, MSc; and Andri Rauch, MD,and the Swiss HIV Cohort Study
Abstract
Background:
Tenofovir-based antiretroviral therapy (ART) has become first-line in all major HIV treatment guidelines. Compared with tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF) has a favorable renal and bone safety profile, but concerns about metabolic complications remain.
Objective:
To assess weight changes, the development of overweight/obesity, and changes in lipid levels 18 months after replacing TDF with TAF.
Design:
Cohort study.
Setting:
5 university hospitals, affiliated hospitals, and private physicians in Switzerland.
Participants:
4375 adults living with HIV who received TDF-containing ART for 6 months or longer.
Measurements:
Changes in weight and lipid levels were assessed using mixed-effect models. Differences in proportions of newly overweight/obese participants were calculated using 2-proportions Z tests.
Results:
4375 individuals were included, with follow-up between 1 January 2016 and 31 July 2019. Median age was 50 years (interquartile range, 43 to 56 years), 25.9% were female, and 51.7% had a normal body mass index (BMI); 3484 (79.6%) switched to TAF and 891 (20.4%) continued TDF.
After 18 months, switching to TAF was associated with an adjusted mean weight increase of 1.7 kg (95% CI, 1.5 to 2.0 kg), compared with 0.7 kg (CI, 0.4 to 1.0 kg) with the continued use of TDF (between-group difference, 1.1 kg [CI, 0.7 to 1.4 kg]).
Among individuals with a normal BMI, 13.8% who switched to TAF became overweight/obese, compared with 8.4% of those continuing TDF (difference, 5.4 percentage points [CI, 2.1 to 8.8 percentage points]).
Switching to TAF led to increases in adjusted mean total cholesterol (0.25 mmol/L [9.5 mg/dL]), high-density lipoprotein cholesterol (0.05 mmol/L [1.9 mg/dL]), low-density lipoprotein cholesterol (0.12 mmol/L [4.7 mg/dL]), and triglyceride (0.18 mmol/L [16.1 mg/dL]) levels after 18 months.
Limitation:
Short follow-up, small subgroup analyses, and potential residual confounding.
Conclusion:
Replacing TDF with TAF is associated with adverse metabolic changes, including weight increase, development of obesity, and worsening serum lipid levels.
Primary Funding Source:
Swiss National Science Foundation.
Background
Tenofovir plays an important role in antiretroviral therapy (ART) for people living with HIV (PLWH) and is recommended as part of the first-line regimens in all major HIV treatment guidelines (1-4). Tenofovir disoproxil fumarate (TDF) has been associated with proximal renal tubulopathy and loss of bone mineral density (5-7). The more favorable bone and renal safety profile of tenofovir alafenamide (TAF) (8-10) led to the replacement of TDF with TAF in most ART guidelines (2-4). However, TAF is not part of the World Health Organization's preferred first-line regimens due to concerns about metabolic side effects (1). In treatment-naive patients, TAF was associated with rising blood lipid levels and an increased need for lipid-lowering therapy compared with TDF (11), and recent data indicate that TAF leads to a substantially larger increase in weight compared with TDF in PLWH initiating ART (12, 13).
So far, weight and metabolic changes have been assessed mainly in ART-naive patients, which makes the interpretation of results challenging. Because effective HIV treatment reduces the infection-associated catabolism, and thereby leads to weight increases in the first months after starting ART (14), it is difficult to differentiate between metabolic changes due to the return to health and adverse drug reactions in individuals initiating ART. In addition, most data on weight and metabolic changes were gathered among selected participants in clinical trials, and data from well-described real-world cohorts are scarce. We used data from the Swiss HIV Cohort Study (SHCS) to assess weight changes and metabolic outcomes in PLWH receiving stable ART who switched from TDF to TAF.
Results
Study Population
Of 10 674 participants under follow-up, 8047 had received TDF for more than 6 months. We excluded 3149 individuals who switched from TDF to a different NRTI, 474 without follow-up after the index visit, and 49 women who became pregnant. The study population included 4375 individuals; the median age was 50 years (interquartile range [IQR], 43 to 56 years), 25.9% were female, and 51.7% had a normal BMI. Of the 4375 participants, 891 (20.4%) continued TDF until the end of the study and 3484 (79.6%) switched to TAF (Appendix Figure 1). Patients who switched to TAF were more likely to be male and men having sex with men, were less likely to be of African origin, had a lower estimated glomerular filtration rate, and were more likely to receive INSTI-based regimens than those who continued TDF (Table 1). The median follow-up was 17.1 months (IQR, 9.6 to 21.3 months) in individuals who switched to TAF and 17.5 months (IQR, 13.0 to 21.2 months) in those who continued TDF. Individuals in both groups were assessed at a median of 3 follow-up visits after the index visit, and 20 (2.2%) who continued TDF and 60 (1.7%) who switched to TAF were lost to follow-up. Observations were missing in less than 2% of visits for all covariates (Appendix Table 1).
Changes in Weight
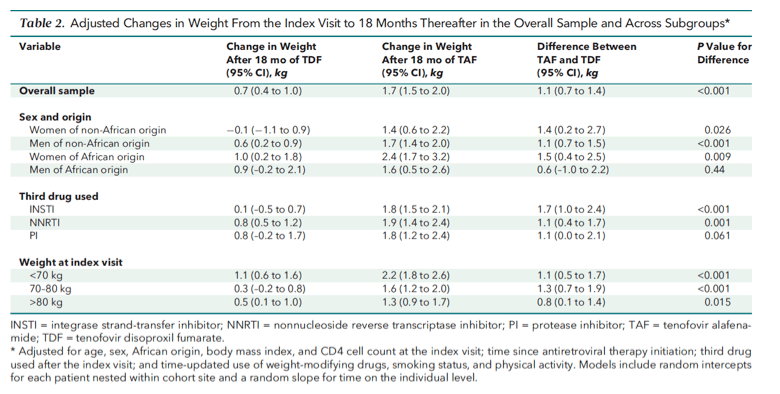
Crude weight trajectories were similar before the index visit and diverged thereafter (Figure 1, top; Appendix Figure 2). In unadjusted analyses, switching to TAF was associated with a mean weight increase of 1.8 kg (95% CI, 1.6 to 2.0 kg) 18 months after the index visit, compared with 0.7 kg (CI, 0.4 to 1.0 kg) with the continuous use of TDF (between-group difference, 1.1 kg [CI, 0.7 to 1.4 kg]). These estimates remained similar after adjustment for confounders (Table 2; Figure 1, middle; Appendix Table 2 and Appendix Figure 3). Figure 1 (bottom) describes changes in BMI categories at 18 months compared with the index visit in all patients with available weight measurements. Among individuals with a normal BMI at the index visit and available follow-up at 18 months, 211 of 1529 (13.8%) who switched to TAF became overweight or obese after 18 months, compared with 35 of 419 (8.4%) who continued TDF (difference, 5.4 percentage points [CI, 2.1 to 8.8 percentage points]).
The use of TAF was associated with statistically significant increases in adjusted mean weight regardless of sex or origin (Table 2). Between-group weight differences of TAF compared with TDF were most pronounced among women of African origin (1.5 kg [CI, 0.4 to 2.5 kg]), followed by women of non-African origin (1.4 kg [CI, 0.2 to 2.7 kg]) and men of non-African origin (1.1 kg [CI, 0.7 to 1.5 kg]), and were not significant among men of African origin (P < 0.001 for the joint interaction with African origin and sex). Weight increases while receiving TAF were observed regardless of the third drug used after the index visit (P = 0.055 for interaction [Table 2]), and the magnitude of weight increases diminished with higher index visit weight and BMI (Figure 2; Table 2; Appendix Figure 4).
Sensitivity Analyses of Weight Changes
Analyses based on individuals with at least 6 and 12 months of follow-up showed similar weight changes as the main analysis (Appendix Table 3). In an analysis restricted to individuals with continuously suppressed HIV viral load, switching to TAF remained associated with an adjusted weight increase of 1.8 kg (CI, 1.6 to 2.1 kg), compared with 0.5 kg (CI, 0.2 to 0.8 kg) in those continuing TDF (Appendix Figure 5). When including only individuals who had TAF replaced by TDF without further changes in ART, TAF remained associated with significant increases in weight, regardless of the third drug used (Appendix Table 4). Finally, switching from ABC to TAF was associated with a weight increase of 1.7 kg (CI, 1.0 to 2.4 kg) after 18 months, compared with 0.5 kg (CI, 0.3 to 0.7 kg) with the continuous use of ABC (between-group difference, 1.2 kg [CI, 0.5 to 1.9 kg]) (Figure 3).
Lipid and Glucose Levels
At the index visit, triglyceride levels and total holesterol-HDL ratios were slightly higher in individuals who switched to TAF than in those who continued TDF, whereas total cholesterol, HDL cholesterol, and LDL cholesterol levels were similar between groups (Table 1). Observations were missing in less than 2.5% of all follow-up visits (Appendix Table 1). Eighteen months after the index visit, switching to TAF was associated with increases in total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride levels, whereas decreases in total cholesterol and LDL cholesterol levels were observed with the continuous use of TDF (Figure 4; Appendix Table 5). During follow-up, 127 individuals (3.6%) who switched to TAF started a new lipid-lowering treatment, compared with 30 (3.4%) who continued using TDF (difference, -0.3 percentage point [CI, -1.7 to 1.1 percentage points]).
Among 4151 individuals without diabetes at the index visit, 4150 (99.9%) contributed to 5616 person-years of follow-up. The crude incidence rate of new-onset diabetes among individuals who switched to TAF was 1.1 per 100 person-years compared with 0.9 per 100 person-years among those who continued TDF (unadjusted incidence rate ratio, 1.2 [CI, 0.6 to 2.6]). After adjustment for age, sex, African origin, and BMI at the index visit, the incidence rate ratio for new-onset diabetes was 1.3 (CI, 0.7 to 2.8) (Appendix Table 6). There was no evidence that switching from TDF to TAF increased incidence of diabetes among individuals with a higher BMI at the index visit (P for interaction = 0.95) (Appendix Figure 6).
Discussion
In this nationwide cohort study, individuals switching from TDF to TAF experienced a larger weight increase than those who continued TDF over 18 months of follow-up. The largest difference between groups was observed among women of African (1.5 kg) and non-African (1.4 kg) origin. Compared with individuals who continued TDF, those who switched to TAF were more likely to become overweight and to experience worsening of serum lipid levels. Our estimates were robust across subgroups of patients regardless of whether TAF was administered together with PIs, NNRTIs, or INSTIs. Taken together, our results highlight the need for continuous monitoring of metabolic comorbid conditions during TAF-containing ART and for further exploring the mechanisms leading to metabolic changes in this population.
Compared with previous publications (identified by an English-language MEDLINE search to "[TAF or tenofovir alafenamid*] AND HIV AND weight"), the weight increase observed in our study is in line with 2 retrospective studies of patients switching from TDF to TAF (20, 21). However, compared with our study, the sample sizes were small (241 and 305, respectively), and the analyses were not adjusted for important confounders. In our study, switching to TAF compared with continuing TDF translated into a larger proportion of individuals with a normal BMI at the index visit who became overweight or obese during the study period, which might further contribute to increasing obesity rates among PLWH (22). The absolute increase in weight on TAF was largest among women of African origin, a finding that was also shown in a large pooled analysis of 8 clinical trials including 5680 treatment-naive PLWH (13). Whereas the underlying mechanisms remain unclear, women of African origin were also at higher risk for obesity among PLWH in a study from the United Kingdom (23). Although less marked than among women of African origin, weight increases among other demographic groups receiving TAF were statistically significant, generally exceeding 1.5 kg after 18 months.
In our study, weight increase while receiving TAF was observed with the concurrent use of all major third drug classes (PIs, NNRTIs, and INSTIs). Whereas a study in treatment-experienced patients showed similar weight changes regardless of the third drug class (24), studies from treatment-naive patients showed larger weight increases among patients receiving INSTI-based regimens (12, 13). Although an additional influence of other drugs, such as INSTI, cannot be excluded, the consistency of our findings across treatment regimens speaks for the important role of switching from TDF to TAF in driving weight increases. Increases in weight while receiving TAF without other ART components have also been observed in a study evaluating TAF-emtricitabine for HIV preexposure prophylaxis (25). Finally, our observation that switching from ABC to TAF was also associated with weight increases further suggests that the increases seen after the replacement of TDF by TAF cannot only be attributed to stopping TDF.
We observed increased lipid levels among individuals who switched to TAF compared with those who continued TDF. These findings confirm and extend observations from registration trials and cohort studies, which consistently showed worsening lipid profiles (9, 26, 27) and an increased demand for lipid-lowering therapy with TAF (11). Several studies indicate that the increase in lipid levels in individuals switching from TDF to TAF might be attributed to stopping TDF, which has an intrinsic lipid-lowering effect (19). Although weight increase and dyslipidemia can affect insulin resistance, we found no clear evidence for increased rates of new-onset diabetes with the use of TAF during the study. Neither TDF nor TAF itself led to insulin resistance among healthy volunteers (28, 29). However, follow-up data from the ADVANCE trial indicate that the large increases in weight observed with the use of TAF led to increased rates of diabetes (30).
Our study is among the largest to date investigating the effect of switching from TDF to TAF on weight and metabolic outcomes within a well-defined and nationally representative cohort. Assessing these outcomes among treatment-experienced PLWH allowed us to avoid the influence of the return to health, which inherently complicates the interpretation of studies among treatment-naive individuals. We adjusted our analyses for a wide range of confounders, including time-updated physical activity and comedications, and our findings remained robust across several sensitivity analyses, including an analysis among patients who switched from ABC to TAF.
Some limitations of our study should be noted. Follow-up was relatively short to grasp the full effect of TAF on new-onset diabetes and lipid metabolism. In addition, subgroup analyses in specific demographic groups were based on small numbers, which limited our ability to detect differences. Information on physical activity and use of weight-modifying drugs was self-reported, and misclassification of these covariates may have affected our results. Although most individuals had replaced TDF with TAF at a time when no published evidence for a treatment-associated weight increase was available, confounding by indication cannot be fully excluded. Assuming that individuals who were more prone to weight increase continued TDF to avoid metabolic side effects of TAF, the difference in weight increase between TDF and TAF would have been underestimated. Third drug classes at the index visit differed markedly between groups, and our methods might have been insufficient to fully adjust for these imbalances. However, a sensitivity analysis restricted to individuals who only replaced TDF with TAF without further ART modifications showed results consistent with our primary analysis. Finally, unmeasured residual confounding cannot be excluded in this observational study. Accordingly, weight increases after switching from TDF to TAF should also be explored in large-scale randomized controlled trials with sufficient follow-up.
In conclusion, our results indicate that switching from TDF to TAF is associated with metabolic adverse events, including obesity and dyslipidemia. Recommendations on the use of TAF should balance its advantages (renal and bone safety) with its potential harms, including metabolic complications. The decision to prefer TAF over TDF as a component of ART should be individualized and accompanied by the repeated assessment of cardiometabolic risk factors, including weight and lipids. Further studies are needed to provide more insight into the mechanisms of weight increase and metabolic effects of modern HIV drugs, to identify individuals at highest risk for such metabolic complications, and to assess the effect of these metabolic complications on clinical outcomes.
|
|
| |
| |
|
|
|