| |
Task Force Issues Draft Recommendation Statement on
Aspirin Use to Prevent Cardiovascular Disease
|
| |
| |
Download the PDF here
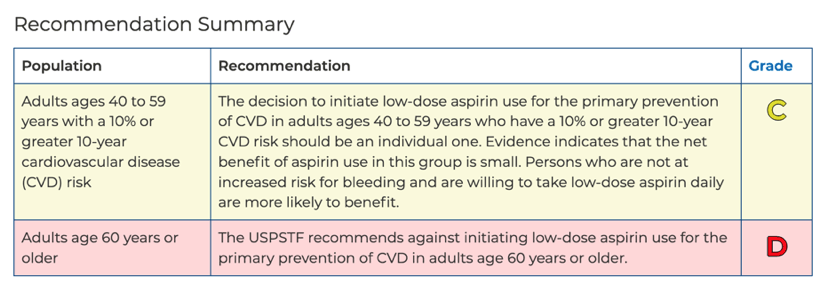
People 40 to 59 should decide with their clinician whether to start taking aspirin;
people 60 or older should not start taking aspirin
WASHINGTON, D.C. - October 12, 2021 - The U.S. Preventive Services Task Force (Task Force) today posted a draft recommendation statement on aspirin use to prevent heart disease and stroke, also known as cardiovascular disease (CVD). People ages 40 to 59 who are at higher risk for CVD and do not have a history of CVD should decide with their clinician whether to start taking aspirin. This is a C grade. People age 60 or older should not start taking aspirin for heart disease and stroke prevention. This is a D grade.
Heart disease and stroke are leading causes of mortality in the United States, accounting for about one in
three deaths. While daily aspirin use has been shown to lower the chance of having a first heart attack or stroke, it can also cause harm. The most serious potential harm is bleeding in the stomach, intestines, and brain. The chance of bleeding increases with age and can be life-threatening.
Based on new evidence since the 2016 Task Force recommendation, it is now recommended that once people turn 60 years old, they should not consider starting to take aspirin because the risk of bleeding cancels out the benefits of preventing heart disease. The latest information also shows a closer balance of benefits and harms than previously understood for people in their 50s and that starting aspirin use as young as 40 years old may have some benefit.
"Daily aspirin use may help prevent heart attacks and strokes in some people, but it can also cause potentially serious harms, such as internal bleeding," says Task Force member John Wong, M.D. "It's important that people who are 40 to 59 years old and don't have a history of heart disease have a conversation with their clinician to decide together if starting to take aspirin is right for them."
This recommendation only applies to people who are at higher risk for CVD, have no history of CVD, and are not already taking daily aspirin. When deciding whether patients should start taking aspirin to prevent a first heart attack or stroke, clinicians should consider age, heart disease risk, and bleeding risk. It is also important to consider a patient's values and preferences. If someone is already taking aspirin and has any questions, they should talk to their clinician about their individual circumstances.
"The latest evidence is clear: starting a daily aspirin regimen in people who are 60 or older to prevent a first heart attack or stroke is not recommended," says Task Force member Chien-Wen Tseng, M.D., M.P.H., M.S.E.E. "However, this Task Force recommendation is not for people already taking aspirin for a previous heart attack or stroke; they should continue to do so unless told otherwise by their clinician."
The Task Force's draft recommendation statement, draft evidence review, and draft modeling report have been posted for public comment on the Task Force website at www.uspreventiveservicestaskforce.org. Comments can be submitted from October 12, 2021, to November 8, 2021, at
www.uspreventiveservicestaskforce.org/tfcomment.htm.
The Task Force is an independent, volunteer panel of national experts in prevention and evidence-based medicine that works to improve the health of people nationwide by making evidence-based recommendations about clinical preventive services such as screenings, counseling services, and preventive medications.
Dr. Wong is interim chief scientific officer, vice chair for Clinical Affairs, chief of the Division of Clinical Decision Making, and a primary care clinician in the Department of Medicine at Tufts Medical Center. He is also director of comparative effectiveness research for the Tufts Clinical Translational Science Institute and a professor of medicine at Tufts University School of Medicine.
Dr. Tseng is the Hawaii Medical Service Association endowed chair in health services and quality research, a professor, and the research director in the Department of Family Medicine and Community Health at the University of Hawaii John A. Burns School of Medicine. She is also a physician investigator with the nonprofit Pacific Health Research and Education Institute.
Contact: USPSTF Media Coordinator at Newsroom@USPSTF.net / (301) 951-9203
Evidence Synthesis
Aspirin Use to Prevent Cardiovascular Disease and
Colorectal Cancer: An Evidence Update for the U.S.
Preventive Services Task Force
Structured Abstract
Background: Cardiovascular disease (CVD) is the leading cause of death and colorectal cancer (CRC) is the third leading cause of death in the United States.
Purpose: To systematically review evidence for the effectiveness of aspirin to prevent myocardial infarction (MI), stroke, cardiovascular death, and all-cause mortality in those without a history of CVD. In addition, to review evidence for CRC incidence and mortality associated with aspirin use in primary and secondary CVD populations. To further review harms associated with aspirin use.
Data Sources: We searched MEDLINE, PubMed, and the Cochrane Collaboration Registry of Controlled Trials to identify literature that was published between January 2014 and January 14, 2021. We supplemented our searches with reference lists from the previous review, relevant existing systematic reviews, suggestions from experts, and Clinicaltrials.gov to identify ongoing trials. We conducted ongoing surveillance for relevant literature through March 26, 2021.
Study Selection: Two investigators independently reviewed identified abstracts and full text articles against a set of a priori inclusion and quality criteria.
Data Analysis: One investigator abstracted data into an evidence table and a second investigator checked these data. We conducted Peto fixed effects meta-analyses to estimate the effect size of aspirin in preventing MI, stroke, CVD-related death and all-cause mortality,
CRC incidence and mortality, major bleeding, major gastrointestinal (GI) bleeding, intracranial bleeding, hemorrhagic stroke, and extracranial bleeding. Additionally, we conducted sensitivity analyses using Mantel-Haenszel fixed effects.
Results: We included 13 fair- to good-quality randomized, controlled trials (RCTs) (N=161,680) examining the effectiveness of aspirin for the primary prevention of CVD. Based on pooled analysis of 11 primary CVD prevention trials using aspirin ≤100 mg/day, low-dose aspirin reduces the risk of major CVD events (total MI, total stroke, CVD mortality) by 10 percent
(k=11, N=134,470; Peto odds ratio [OR], 0.90 [95% confidence interval (CI), 0.85 to 0.95]), MI by 11 percent (k=11, N=134,470; Peto OR, 0.89 [95% CI, 0.82 to 0.96]), and ischemic stroke by 18 percent (k=5, N=79,334; Peto OR, 0.82 [95% CI, 0.72 to 0.92]) with no differences in CVD mortality (k=11, N=134,470; Peto OR, 0.95 [95% CI, 0.86 to 1.05]) or all-cause mortality (k=11, N=134,470; Peto OR 0.98 [95% CI, 0.93 to 1.03]). Absolute risk reductions in major CVD events in the trials ranged from 0.08 to 2.5 percent. Aspirin's benefits were similar when trials of all doses were pooled. Sensitivity analyses restricted to more recent trials where usual care includes aggressive risk factor modification including statin therapy show diminished effects of aspirin for major CVD events and total MI but larger effects for total ischemic stroke compared to older trials. A small subset of the trials reporting CVD outcomes also reported CRC outcomes. Based on 4 low-dose aspirin trials (N=86,137) recruiting primary CVD prevention populations, there was no statistically significant association between aspirin and CRC incidence when analyzing randomized trial periods (Peto OR 1.07 [95% CI, 0.92 to 1.24]; trial period 5-10 years). Analysis including post-trial observation periods up to 20 years and including trials with high-dose aspirin up to 500 mg/day (k=2; N=45,015) in primary prevention populations show statistically significant reductions in CRC incidence (0.70 [95% CI, 0.50 to 0.98] and 0.82 [95% CI, 0.69 to 0.98]). Two low-dose aspirin RCTs (N=59,020) in primary CVD prevention populations report CRC mortality during the trial period (5-10 years) showing results concerning for possible harm with one trial demonstrating a statistically significant increase in CRC mortality in older adults. At 18 years of followup, including post-trial observational periods, three primary CVD prevention trials with mean daily aspirin doses ranging from 75 to 500 mg showed aspirin was associated with a decreased risk of CRC mortality (Peto OR 0.76 [95% CI, 0.62 to 0.94]). Low-dose aspirin is associated with a 31 percent increase in intracranial bleeding events (k=11; N=134,470; Peto OR, 1.31 [95% CI, 1.11 to 1.54]), and 53 percent increase in extracranial bleeding events (k=10; N=133,194; Peto OR 1.53 [95% CI, 1.39 to 1.70]). The absolute increases ranged from -0.2 to 0.4 percent for intracranial bleeding events and 0.2 to 0.9 percent for extracranial bleeding events.
There is no compelling evidence to suggest that aspirin has a different relative CVD benefit or bleeding risk in specific populations defined by age, sex, race and ethnicity, diabetes status, or baseline 10-year CVD risk. Aspirin's CVD benefits appear to begin within the first 1-2 years of administration and the bleeding harms begin soon after aspirin initiation; there are limited data for more precise time increments or longer durations.
Limitations: Primary CVD prevention trials used different aspirin doses in heterogeneous populations with relatively short study followup, with duration mostly ranging from 4-6 years. Trials reporting CRC incidence and mortality outcomes are limited by short trial duration; observational followup of trials are limited by heterogeneity of aspirin doses, duration, indications, and populations with risk of biases and confounding. Estimates of rare bleeding harms are imprecise.
Conclusions: In primary prevention populations, low-dose aspirin reduces major CVD events, MI and ischemic stroke, but also increases major GI bleeding, extracranial bleeding, and intracranial bleeding. Our evidence suggests aspirin is associated with a possible long-term reduction in CRC incidence and mortality based on post-trial period observation, but the results are limited for low-dose aspirin among primary CVD prevention populations. More precise real-world U.S.-based estimates for bleeding events in the general population and specific populations with elevated CVD risk are necessary to accurately estimate the net benefit. Depending on CVD risk, this absolute CVD benefit in specific populations could potentially outweigh the bleeding risks. Models to identify these populations are needed.
|
|
| |
| |
|
|
|