| |
Initiation of long-acting cabotegravir plus rilpivirine as
direct-to-injection or with an oral lead-in in adults with
HIV-1 infection: week 124 results of the open-label phase 3 FLAIR study
|
| |
| |
Download the PDF here
Introduction
Contemporary antiretroviral therapy (ART) has revolutionised HIV treatment, with sustained virological suppression an achievable target for nearly all people with HIV with access to ART. Guideline-recommended regimens generally consist of one or two nucleoside reverse transcriptase inhibitors (NRTIs) in combination with an integrase strand transfer inhibitor (INSTI), a non-nucleoside reverse transcriptase inhibitor (NNRTI), or a boosted protease inhibitor.1, 2
Current regimens require strict adherence to daily oral therapy for sustained virological suppression. Poor adherence, which results in insufficient drug concentrations, is associated with virological failure, emergent resistance, and worse outcomes.3, 4, 5
Because modern regimens are highly effective, the emphasis of HIV care has shifted from solely efficacy to quality of life improvements through treatment simplification, better tolerability, and patient satisfaction, which can positively influence adherence. These drivers have led to the development of two-drug oral regimens, with the aim of reducing cumulative drug exposure compared with standard three-drug therapy.6, 7
People with HIV have expressed increasing interest in long-acting ART to afford greater convenience with daily routines and travel,8, 9, 10, 11 which together with a reduced dosing frequency might support improved treatment adherence.12, 13 Furthermore, long-acting therapy might alleviate the psychological burden of daily oral therapy, including fear of inadvertent disclosure of HIV status and possible stigmatisation.14, 15 Additionally, some people with HIV have reported that daily ART restricts their daily life.
16 This patient-first approach to HIV treatment aims to improve quality of life and health outcomes for people with HIV. 17
------------------------
Reassessing oral lead-in for injectable long-acting HIV therapy
October 14, 2021 Lancet HIV Paul Sax, Josep M Libre
Long-acting antiretrovirals administered as pills, subcutaneous or intramuscular injections, or implants achieve prolonged drug exposures at therapeutic concentrations, circumventing the requirement for taking pills daily. Cabotegravir, rilpivirine, leronlimab, islatravir, albuvirtide, lenacapavir, and broadly neutralising antibodies all offer the potential for this novel therapy.1
As the first US Food and Drug Administration and European Medical Agency approved long-acting complete antiretroviral treatment, cabotegravir plus rilpivirine constitutes a major advance in HIV treatment. With a terminal phase half-life of 6–11 weeks for cabotegravir and 13–28 weeks for rilpivirine, the combination is administered through an extended-release injectable suspension once every month or once every 2 months,2 resulting in detectable drug concentrations up to 48 weeks or more upon treatment discontinuation.3
Cabotegravir plus rilpivirine has not only shown non-inferior virological suppression compared with daily oral therapy, it also proved highly popular with participants in the pivotal clinical trials.
As currently approved, the cabotegravir plus rilpivirine injectable regimen requires a 4 week period of oral therapy before the first injections. The goal of this strategy is to avoid potential adverse effects, mainly hypersensitivity reactions, management of which would be highly challenging because there is no way to accelerate clearance of the drugs once administered. The oral lead-in complicated the design of clinical trials and now implementation of this treatment in clinical practice. It also forces drug manufacturers to produce packages solely for oral cabotegravir and rilpivirine lead-in periods, a treatment that will only continue for 4 weeks.
Of note, there is precedent for long-acting drugs without oral lead-in. Lead-in is not required for some long-acting antipsychotic drugs for schizophrenia (eg, aripiprazole and fluphenazine decanoate), nor do long-acting contraceptives require any previous oral lead-in. An HIV therapy already exists that does not have an oral formulation and no requirement for an oral lead-in, the monoclonal antibody ibalizumab.
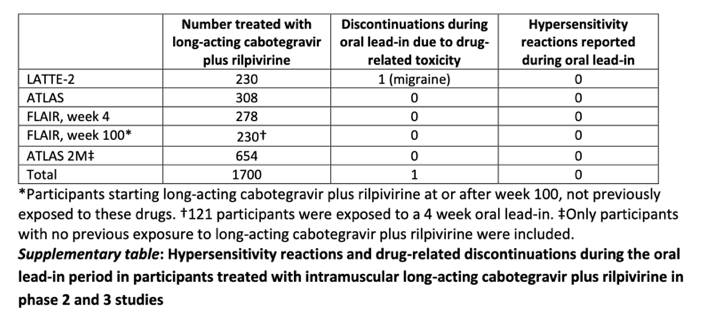
For cabotegravir and rilpivirine, no major safety signals and no hypersensitivity reactions were identified during the oral lead-in period across preclinical studies and the phase 2 and 3 programme development (1470 participants; appendix).3, 4, 5, 6, 7, 8 These encouraging results provided the rationale to assess switching to injectable cabotegravir plus rilpivirine without the oral lead-in.
In The Lancet HIV, Chloe Orkin and colleagues4 report the 124 week data of the phase 3 FLAIR study, which included a subset of participants with HIV who were offered and chose the opportunity to switch from standard oral to long-acting therapy directly. The decision was made by the participant after discussion with the investigator and hence was not a randomised comparison, but the groups that opted for each method were similar.
At week 100, 232 (92%) of 253 study participants transitioned to long-acting cabotegravir plus rilpivirine in the extension phase, 111 (48%) participants did not have an oral lead-in and 121 (52%) of participants opted for the standard 4 week oral lead-in. No serious adverse events or drug-related events leading to withdrawal occurred during the first 4 weeks of transition and no drug-related hypersensitivity reactions were documented and no participant met protocol-defined liver stopping criteria.
The results showed that initiating cabotegravir plus rilpivirine without an oral lead-in had similar safety to initiation following an oral lead-in, with no effect on efficacy. The study includes an elegant pharmacokinetic analysis that compares cabotegravir and rilpivirine concentrations from oral and intramuscular administration at 1 week and 4 weeks after first doses, and, reassuringly, finds no clinically meaningful differences.
Starting long-acting injectable cabotegravir and rilpivirine without the need for an oral lead-in would undoubtedly be more convenient for those choosing this novel therapy. Regulatory agencies will now review existing data to assess whether these safety data are sufficient to warrant a change in prescribing information. Meanwhile, the present analysis builds upon the existing compiled experience with approximately 1500 people treated in phase 2 and 3 trials, and it suggests that a mandatory oral lead-in could be an overabundance of caution and a needless strategy; treating ourselves for reassurance rather than treating our patients.
JML reports consulting honoraria from ViiV Healthcare, Gilead Sciences and Janssen-Cila; and research grants from Gilead Sciences and ViiV Healthcare. PES has served on advisory boards and received grants and personal fees from Gilead, ViiV Healthcare, Janssen, and Merck.
----------------------------
Initiation of long-acting cabotegravir plus rilpivirine as direct-to-injection or with an oral lead-in in adults with HIV-1 infection: week 124 results of the open-label phase 3 FLAIR study
October 14, 2021 Lancet HIV - Chloe Orkin, Enrique Bernal Morell, Darrell H S Tan, Harold Katner, Hans-Jürgen Stellbrink, Elena Belonosova, Rebecca DeMoor, Sandy Griffith, Shanker Thiagarajah, Rodica Van Solingen-Ristea, Susan L Ford, Herta Crauwels, Parul Patel, Amy Cutrell, Kimberly Y Smith, Kati Vandermeulen, Eileen Birmingham, Marty St Clair, William R Spreen, Ronald D’Amico
Summary
Background
Previous work established non-inferiority of switching participants who were virologically suppressed from daily oral standard of care to monthly long-acting intramuscular injections of cabotegravir plus rilpivirine over 96 weeks following a cabotegravir plus rilpivirine oral lead-in. Here, we report an evaluation of switching participants from standard of care oral regimens to long-acting cabotegravir plus rilpivirine via direct-to-injection or oral lead-in pathways.
Methods
This study reports the week 124 results of the FLAIR study, an ongoing phase 3, randomised, open-label, multicentre (11 countries) trial. Antiretroviral therapy (ART)-naive participants who were virologically suppressed (HIV-1 RNA <50 copies per mL) during the 20-week induction phase with standard of care were randomly assigned (1:1) to continue the standard of care oral regimen or switch to long-acting cabotegravir plus rilpivirine (283 per group) in the 100-week maintenance phase. Randomisation was stratified by sex at birth and baseline (pre-induction) HIV-1 RNA (<100 000 or ≥100 000 copies per mL). Participants randomly assigned to long-acting therapy at baseline received a cabotegravir (30 mg) plus rilpivirine (25 mg) once daily oral lead-in for at least 4 weeks before first injection and could choose to continue long-acting cabotegravir (400 mg) plus rilpivirine (600 mg) every 4 weeks from week 100 or withdraw. At week 100, participants in the oral comparator ART group, in discussion with the investigator, could elect to switch to long-acting therapy (extension switch population), either direct-to-injection or with a 4 week oral lead-in (oral lead-in group), or withdraw. Week 124 endpoints included plasma HIV-1 RNA 50 or more copies per mL and less than 50 copies per mL (US Food and Drug Administration [FDA] Snapshot), confirmed virological failure (two consecutive HIV-1 RNA ≥200 copies per mL), and safety and tolerability. The study is registered at ClinicalTrials.gov, NCT02938520.
Findings
Screening occurred between Oct 27, 2016, and March 24, 2017. At week 100, 232 (92%) of 253 participants transitioned to long-acting cabotegravir plus rilpivirine in the extension phase (111 [48%] in the direct-to-injection group and 121 [52%] in the oral lead-in group; extension switch population). 243 (86%) of the 283 who were randomly assigned to the long-acting therapy group continued the long-acting regimen into the extension phase. One (<1%) participant in each extension switch group had 50 or more HIV-1 RNA copies per mL; 110 (99%) participants in the direct-to-injection group and 113 (93%) participants in the oral lead-in group remained suppressed (HIV-1 RNA <50 copies per mL) at the week 124 Snapshot. The lower suppression rates in the oral lead-in group were driven by non-virological reasons. For participants in the randomly assigned long-acting group, 227 (80%) of 283 participants remained suppressed; at the week 124 Snapshot, 14 (5%) participants had HIV-1 RNA 50 or more copies per mL, including five additional participants since the week 96 analysis. The remaining 42 (15%) participants in the randomly assigned long-acting group had no virological data. Adverse events leading to withdrawal were infrequent, occurring in three (1%) participants in the extension switch population (one in the direct-to-injection group and two in the oral lead-in group) after 24 weeks of cabotegravir plus rilpivirine therapy, and 15 (5%) participants in the randomly assigned long-acting group up to 124 weeks of therapy. No deaths occurred in the extension phase. Overall, cabotegravir plus rilpivirine adverse event type, severity, and frequency were similar across all groups. Injection site reactions were the most common adverse event, occurring after 914 (21%) of 4442 injections in the extension switch population and 3732 (21%) of 17 392 injections in the randomly assigned long-acting group. Injection site reactions were mostly classified as mild-to-moderate in severity and decreased in incidence over time. Four (2%) of 232 participants in the extension switch population and seven (2%) of 283 in the randomly assigned long-acting group withdrew due to injection-related reasons.
Interpretation
After 24 weeks of follow-up, switching to long-acting treatment with or without an oral lead-in phase had similar safety, tolerability, and efficacy, supporting future evaluation of the simpler direct-to-injection approach. The week 124 results for participants randomly assigned originally to the long-acting therapy show long-acting cabotegravir plus rilpivirine remains a durable maintenance therapy with a favourable safety profile.
Funding
ViiV Healthcare and Janssen Research & Development.
One (1%) of the 111 participants in the direct-to-injection group met the confirmed virological failure criterion during the first 24 weeks of cabotegravir plus rilpivirine therapy. The participant had suspected virological failure (first HIV-1 RNA ≥200 copies per mL) at week 112 following three monthly injection doses, with a viral load of 348 copies per mL, followed by a viral load of 1218 copies per mL at the subsequent confirmatory visit. The participant was male (sex at birth), from the USA, had a BMI of 30 kg/m2 or more, and had HIV-1 subtype B; no INSTI or NNRTI resistance-associated mutations (RAMs) were detected at induction baseline (week −20). At the time of suspected virological failure, no INSTI RAMs were detected and the participant had full phenotypic susceptibility to cabotegravir and dolutegravir; PhenoSense (Monogram Biosciences, South San Francisco, CA, USA) genotype could not be generated. The participant had plasma cabotegravir and rilpivirine concentrations below the fifth percentiles (cabotegravir 0·349 μg/mL; rilpivirine 16·4 ng/mL) 4 weeks following initiating injections (appendix p 3).
There were no drug-related hypersensitivity reactions and no significant creatinine changes from baseline for the extension switch or randomly assigned long-acting groups since the week 96 analysis. There were no clinically significant changes in lipase concentration in the extension switch population (from extension baseline) or the randomly assigned long-acting group; no lipase abnormalities were associated with clinical pancreatitis diagnoses. One (1%) participant in the direct-to-injection group, one (1%) in the oral lead-in group, and two (1%) in the randomly assigned long-acting group (since the week 96 analysis) had alanine aminotransferase concentrations three or more times higher than the upper limit of normal (single episodes each). No participants in the oral lead-in or direct-to-injection groups and only one (<1%) participant in the randomly assigned long-acting group met protocol-defined liver stopping criteria. This participant met liver stopping criteria at week 124 due to secondary syphilis and was treated with penicillin; after completing the treatment course, the participant's alanine aminotransferase and aspartate aminotransferase concentrations normalised. The case was adjudicated by the study sponsor who approved restarting long-acting dosing based on a clear cause and no indications of drug-induced liver injury. There were no cases of drug-induced liver injury in the extension switch population. During the extension phase at week 112, one (<1%) participant in the extension switch population (oral lead-in group) had a grade 1 adverse event: weight gain (progressive weight gain of 8 kg). This adverse event was assessed as study drug related and led to withdrawal. Another participant in the randomly assigned long-acting group had an adverse event of weight gain since the 96-week analysis. This was classified as grade 1 and not related to study drug.
Neither of the two participants who received long-acting cabotegravir plus rilpivirine who met the confirmed virological failure criterion during the extension phase had any INSTI or NNRTI mutations detected at baseline. The additional participant in the randomly assigned long-acting group with confirmed virological failure since the week 96 analysis had the integrase polymorphism Leu74Ile at baseline, consistent with three participants in this group who met the confirmed virological failure criterion during the maintenance phase while on long-acting therapy.19 A recently published post-hoc multivariable analysis using a pooled population from the FLAIR, ATLAS, and ATLAS-2M studies found that the presence of two or more of the following baseline factors increased confirmed virological failure risk: baseline proviral rilpivirine RAMs, subtype A6 or A1, and a BMI of 30 kg/m2 or more.26 The role of these three factors in confirmed virological failure with long-acting cabotegravir plus rilpivirine has been extensively discussed previously.26 The two participants who met confirmed virological failure in the extension phase of this study had one of the three factors each: one had a BMI of 30 kg/m2 or more and the other had subtype A6. Both participants were resuppressed on alternate regimens.
|
|
| |
| |
|
|
|