| |
Excess Weight Gain With Integrase Inhibitors and Tenofovir Alafenamide:
What Is the Mechanism and Does It Matter?
|
| |
| |
Download the PDF here
Open Forum Infectious Diseases, December 2021
Brian R. Wood1 and Gregory D. Huhn2 - 1Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA, 2Division of Infectious Diseases, Cook County Health, Chicago, Illinois, USA, and 3Division of Infectious Disesases, Rush University Medical Center, Chicago, Illinois, USA
https://academic.oup.com/ofid/article/8/12/ofab542/6430220
Abstract
Numerous studies have detected a greater likelihood of excess weight gain with specific antiretrovirals (ARVs), particularly tenofovir alafenamide and integrase inhibitors, as compared with other agents and classes. The long-term implications and potential reversibility for individuals who have experienced substantial ARV-associated weight accumulation remain poorly understood. Furthermore, the underlying mechanism remains controversial: Is the explanation mitochondrial toxicity and weight suppression from the older agents or direct effects of the newer drugs on appetite, adipocytes, or other unintended targets? This review discusses proposed mechanisms and evidence to date and argues that the question about mechanism is highly clinically relevant because it carries significant implications for ARV management. The existing literature suggests that older ARVs, such as tenofovir disoproxil fumarate and efavirenz, suppress weight gain, but also that integrase inhibitors may stimulate excess weight gain through several plausible biologic pathways. Confirming the mechanisms of ARV-associated excess weight gain should be high priority for future research.
Summary of Data on TAF and Weight Gain Mechanisms
Integrating the data from ART initiation, switch, and PrEP trials, we find differential likelihood of weight change with various NRTIs. While direct effects of TAF on weight have not been fully excluded and require further study, the preponderance of findings to date suggest that the primary explanation is a continuum of weight suppressive effects within the NRTI class, such that the NRTI backbone at ART initiation or pre-ART switch affects the likelihood of excess weight accumulation. The primary limitation is that the findings to date rely on clinical trial and retrospective observational comparisons. Unlike with INSTIs, as we will discuss, we are unable to identify laboratory studies assessing the effects of TAF vs other NRTIs on hormones that control appetite or regulate weight; such laboratory studies would be valuable to the field.
Summary of Data on INSTIs and Weight Gain Mechanisms
The constellation of findings on INSTIs and weight gain mechanisms is different than that for TAF. For the INSTIs, several plausible biologic mechanisms for direct effects have been proposed. The literature to date suggests that the preswitch anchor drug plays a role and that older anchor drugs like EFV likely suppress weight, particularly for individuals with certain genetic profiles, but also that INSTIs may directly stimulate weight accumulation through several proposed pathways. Future research should assess these specific pathways, clarify whether this is an INSTI class effect or is unique to the newer INSTIs, and continue to examine the long-term clinical consequences as well as pros vs cons of switching an INSTI to an alternative option.
Investigators assessed weight gain 48 weeks after switch from EFV to an INSTI in 2 cohorts (61 individuals from an observational cohort and 462 from a clinical trial cohort) and evaluated whether the CYP2B6 and UGT1A1 genotypes were associated with weight change following the ART switch (both genotypes are associated with ART metabolism and drug levels) [37]. Interestingly, the results demonstrated that CYP2B6 EFV slow metabolizers experienced significantly greater weight gain after the switch from EFV to EVG or RAL, but not DTG. In the pooled analysis of data from multiple clinical trials that enrolled PWH with suppressed viral loads and randomized participants to maintain baseline ART or switch, the greatest risk of excess weight gain (>10% by week 48) occurred with a switch from EFV to RPV or EVG/c. Participants switching off EFV or TDF experienced the greatest weight gain [47]. From these trials, it appears that EFV suppresses weight gain, especially for individuals with pharmacogenomic profiles associated with higher drug levels, and this influences weight change after switch to a different NNRTI or an INSTI.
CONCLUSIONS
The mechanisms responsible for TAF- and INSTI-associated excess weight gain remain incompletely understood. Our interpretation of the literature to date is that the mechanism of excess weight gain from TAF and INSTIs is most likely different, credible theories about direct effects from INSTIs exist but must be corroborated, and it is likely that older agents like TDF and EFV suppress weight more than previously realized. Clinical trials like ACTG 5391 [78] will add insight into whether switching from an INSTI to DOR, with or without a switch from TAF to TDF, leads to reversal of weight gain; a case report describes weight loss when switching an INSTI-TAF combination back to TDF with an NNRTI, so this is plausible [22]. Additional trials, like DEFINE, will assess whether switching from an INSTI to a boosted PI is beneficial for individuals who have experienced rapid and significant weight gain while taking an INSTI with TAF; this will add further insights into the pros and cons of such a switch, particularly isolating the outcome of switching off an INSTI as both the intervention and comparator arms will continue taking TAF [79]. However, ongoing research is also needed to confirm the mechanisms and further explore differences based on birth sex and genetic factors. Until we better understand the phenomenon of ARV-associated excess weight gain, clinicians will need to carefully monitor patients for adverse metabolic or cardiovascular outcomes and counsel those who have gained substantial weight while taking TAF and/or an INSTI on healthy lifestyle habits, together with what is known and unknown about the potential benefits and risks of switching ART.
———————————————
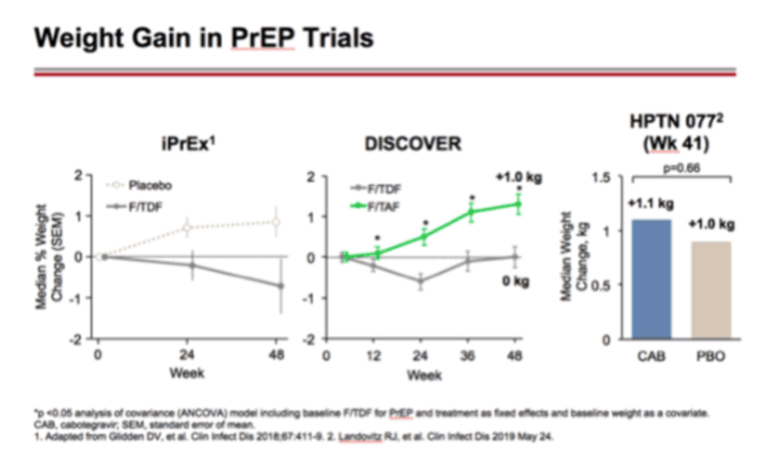
Outcomes of Participants Switching from F/TDF to F/TAF for PrEP: Week 48 Results from the DISCOVER Open Label Phase
Longer Term Efficacy and Safety of F/TAF and F/TDF For HIV PrEP: DISCOVER Trial Week 96 Results - (03/11/20)
PrEP & Lipids, Weight Gain - DISCOVER Study
Weight Change in NEAT-022, Switch to dolutegravir from boosted PI, 96 weeks at Glasgow 2018 - (11/11/21)
CABOTEGRAVIR IS NOT ASSOCIATED WITH WEIGHT GAIN IN HIV-NEGATIVE INDIVIDUALS
Weight Change in - HPTN 083 FINAL RESULTS: Pre-exposure Prophylaxis containing long-acting injectable cabotegravir is safe and highly effective for cisgender men and transgender women who have sex with men
Weight Change Following Antiretroviral Therapy Switch in People With Viral Suppression: Pooled Data from Randomized Clinical Trials
"In summary, our results demonstrate that modest weight gain is common after ART switch and is correlated more strongly with baseline regimen, especially switch off of TDF or EFV, than with sex-, race-, or HIV-related factors. It remains uncertain whether this is due to the loss of a weight suppressive effect of prior regimens or a weight gain effect of the newer regimen. A better understanding of the underlying biological mechanisms and the clinical implications is needed to fully understand these observations. Close monitoring of weight and counseling to maintain a healthy diet and remain physically active, as well as optimize other lifestyle factors, is imperative for all patients on ART [40].
In this pooled analysis of 12 randomized studies of ART switch, we found that weight gain occurred in both switch and SBR participants, with the magnitude of weight gain generally greater with switches to newer regimens, consistent with observations from other ART-naive and switch studies [2, 4-11]. Additionally, baseline ART regimen was a significant predictor of weight gain after switch. For example, in participants who switched to EVG/c, switch from EFV was associated with weight gain, switch from a PI or NVP to EVG/c was weight-neutral, and switch from RPV to EVG/c was associated with weight loss. Among the NRTIs, switch from TDF to TAF was associated with greater weight gain than switch from ABC to TAF. Younger age and lower baseline BMI were associated with weight gain, while race, ethnicity, sex, and CD4 count were not."
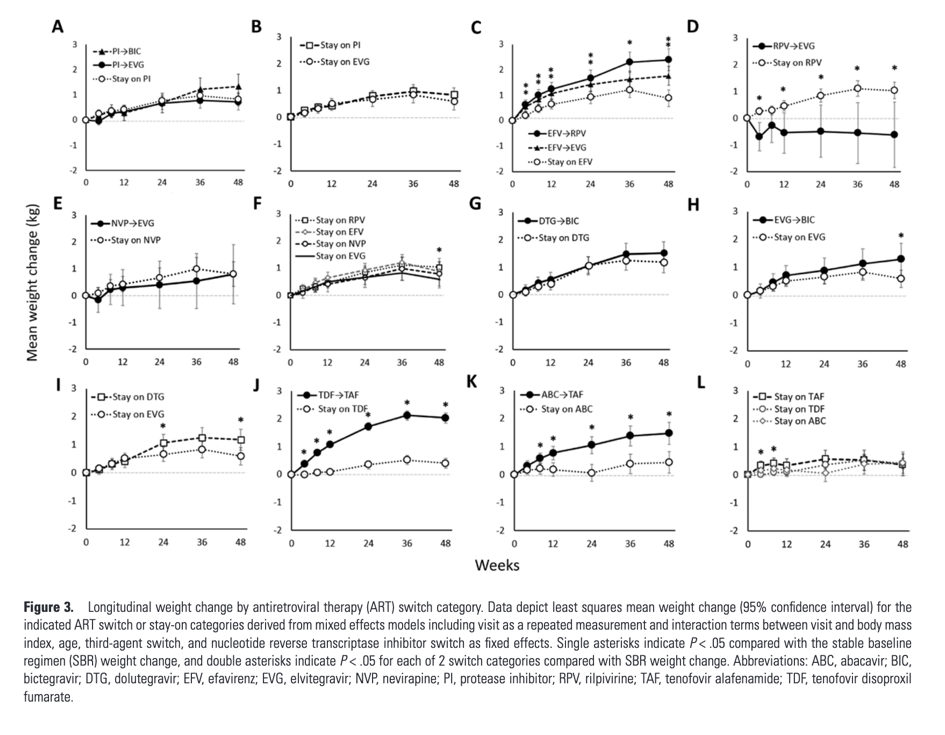
Weight change with switch from boosted PI to elvitegravir/cobicistat (EVG/c) or BIC did not differ significantly from staying on a PI or EVG/c (Figure 3A, 3B). Switch from efavirenz (EFV) to rilpivirine (RPV) or to EVG/c was associated with 1.5 kg and 0.9 kg greater weight gain at week 48, respectively, compared with staying on EFV (both P < .001; Figure 3C), while switch from RPV to EVG/c was associated with 1.7 kg weight loss at week 48 (P = .01; Figure 3D). Switch from nevirapine (NVP) to EVG/c was associated with similar weight gain as staying on NVP (Figure 2E). Similar weight gain was seen between NNRTI SBR categories, with the exception of greater weight gain in stay-on RPV vs stay-on EVG/c (P = .046; Figure 3F). Among those on an INSTI at baseline, weight change with switch from DTG to BIC was not significantly different from remaining on DTG; switch from EVG/c to BIC was associated with a 0.7 kg greater weight gain at week 48 compared with no switch (P = .034; Figure 3G, 3H). Staying on DTG was associated with a 0.6 kg greater weight gain than staying on EVG/c at week 48 (P = .02; Figure 3I). Among NRTI switches, weight gain was seen when switching from tenofovir disoproxil fumarate (TDF; 1.6 kg or abacavir (ABC) to TAF (both P < .001; Figure 3J, 3K). Participants who stayed on TDF or ABC had weight changes similar to those who remained on TAF (Figure 3L).
Factors Associated With ≥10% Weight Gain
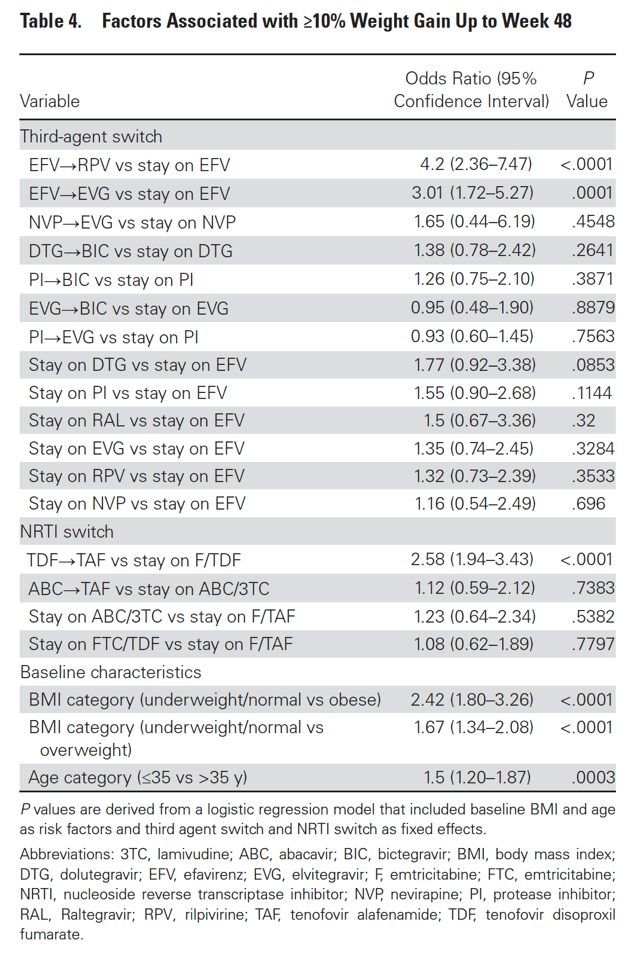
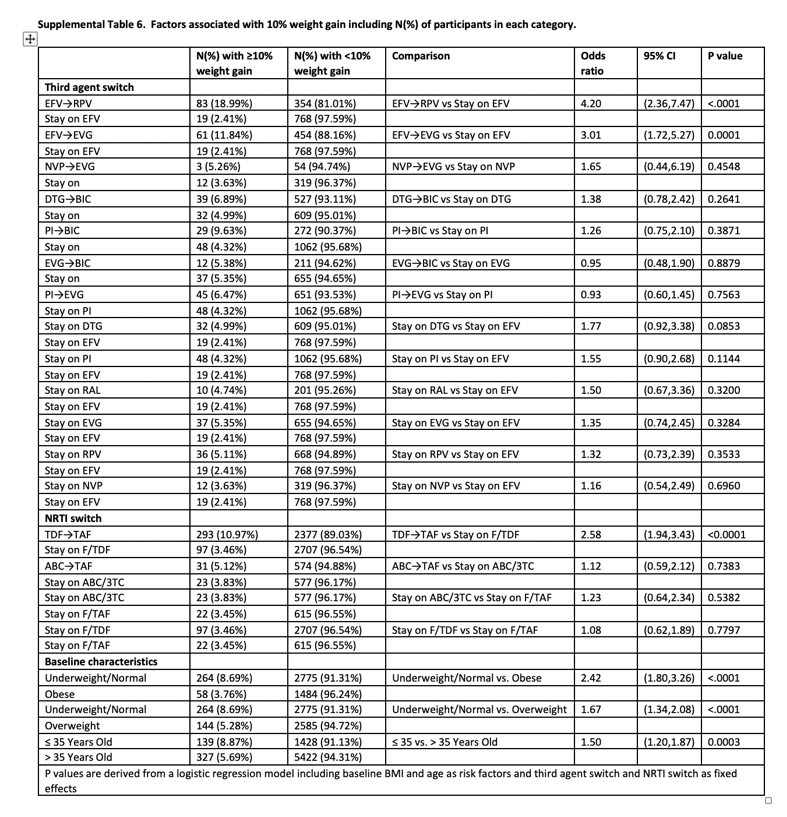
At week 48, 4.6% of participants had ≥10% weight gain (6.4% of switch and 2.2% of SBR). Participants with ≥10% weight gain were younger and had lower baseline weight and BMI; other characteristics were similar between groups (Table 3). Baseline characteristics were generally well balanced between switch and SBR participants within the ≥10% gain and <10% gain groups (Supplementary Tables 4 and 5). In logistic regression models that included demographic and clinical variables as above, only baseline underweight/normal BMI category and age ≤35 years were associated with ≥10% weight gain. In a subsequent logistic regression model also including ART switch categories, there was increased risk for ≥10% weight gain with switch from EFV to RPV or to EVG/c but not with other third agent switches. Among NRTI switch categories, switch from TDF to TAF was associated with ≥10% weight gain, while switch from ABC to TAF was not (Table 4, Supplementary Table 6).
Longitudinal Weight Change by Sex and Race
As the lack of associations between race, sex, and 10% weight gain was unexpected, we performed additional analyses, focusing on sex and race. Females had a 0.3 kg greater weight gain compared with males at week 48 (P = .0046; Figure 4A); Black and non-Black participants did not significantly differ (Figure 4B). In models that assessed sex and race interactions, Black males had 0.3 kg greater weight gain than non-Black males (P = .041); non-Black females had 0.5 kg greater weight gain than non-Black males at week 48 (P = .013; Figure 4C). In contrast, females had similar weight gain regardless of race, and Blacks had a similar weight gain regardless of sex. We further explored the race and sex differences in study 380-1961, a trial that enrolled only women, of whom 37% were Black. Non-Black females (15.7% of whom were Hispanic or Latinx) experienced numerically greater weight gain after switching from TDF to TAF compared with Black females (3.2 vs 1.9 kg, P = .07). A sensitivity analysis that excluded study 380-1961 revealed similar results as for the full cohort (week 48 difference between Black and non-Black females, 0.23 kg, P = .42).
|
|
| |
| |
|
|
|