| |
Week 96 extension results of a Phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment
|
| |
| |
Download the PDF here
Swindells, Susana; Lutz, Thomasb; Van Zyl, Lelaniec; Porteiro, Normad; Stoll, Matthiase; Mitha, Essackf; Shon, Alyssag; Benn, Paulh; Huang, Jenny O.i; Harrington, Conn M.j; Hove, Kaik; Ford, Susan L.l; Talarico, Christine L.j; Chounta, Vasilikih; Crauwels, Hertam; Van Solingen-Ristea, Rodicam; Vanveggel, Simonm; Margolis, David A.j,n; Smith, Kimberly Y.j; Vandermeulen, Katim; Spreen, William R.j
Abstract
Background:
ATLAS (NCT02951052), a phase 3, multicenter, open-label study, demonstrated that switching to injectable cabotegravir (CAB) with rilpivirine (RPV) long-acting dosed every 4 weeks was noninferior at week (W) 48 to continuing three-drug daily oral current antiretroviral therapy (CAR). Results from the W 96 analysis are presented.
Methods and design:
Participants completing W 52 of ATLAS were given the option to withdraw, transition to ATLAS-2M (NCT03299049), or enter an Extension Phase to continue long-acting therapy (Long-acting arm) or switch from CAR to long-acting therapy (Switch arm). Endpoints assessed at W 96 included proportion of participants with plasma HIV-1 RNA less than 50 copies/ml, incidence of confirmed virologic failure (CVF; two consecutive HIV-1 RNA ≥200 copies/ml), safety and tolerability, pharmacokinetics, and patient-reported outcomes.
Results:
Most participants completing the Maintenance Phase transitioned to ATLAS-2M (88%, n = 502/572). Overall, 52 participants were included in the W 96 analysis of ATLAS; of these, 100% (n = 23/23) and 97% (n = 28/29) in the Long-acting and Switch arms had plasma HIV-1 RNA less than 50 copies/ml at W 96, respectively. One participant had plasma HIV-1 RNA 50 copies/ml or higher in the Switch arm (173 copies/ml). No participants met the CVF criterion during the Extension Phase. No new safety signals were identified. All Switch arm participants surveyed preferred long-acting therapy to their previous daily oral regimen (100%, n = 27/27).
Conclusion:
In this subgroup of ATLAS, 98% (n = 51/52) of participants at the Extension Phase W 96 analysis maintained virologic suppression with long-acting therapy. Safety, efficacy, and participant preference results support the therapeutic potential of long-acting CAB+RPV treatment for virologically suppressed people living with HIV-1.
Here, we present results from the W 96 analysis of the Extension Phase of ATLAS, constituted of a subgroup of participants initially randomized into ATLAS who did not transition to the ATLAS-2M study. We report efficacy, safety and tolerability, pharmacokinetic, and patient-reported outcome data for participants who switched from the current oral ART comparator arm (CAR arm) to long-acting CAB+RPV Q4W at the conclusion (W 52) of the Maintenance Phase (Switch arm). In addition, longer term outcomes are reported for participants already randomized to long-acting CAB+RPV Q4W at the start of the Maintenance Phase (Long-acting arm), representing 96 weeks of long-acting therapy.
Safety and tolerability
Safety data collected during the Extension Phase for the Long-acting and Switch arms were comparable with those collected for the Long-acting arm during the Maintenance Phase, with respect to the type of overall adverse events, non-ISR adverse events, and common nonserious adverse events (Table 1 and Supplemental Digital Content Table S1, https://links.lww.com/QAD/C245, an overview of common nonserious adverse events). Overall, 105 and 79 participants in the Switch arm reported adverse events and drug-related adverse events, respectively. Four Switch arm participants reported Grade 3 or above adverse events [Grade 3 injection site pain (n = 3) and Grade 4 increased lipase (n = 1)]. One new participant in the Long-acting arm reported an adverse event during the Extension Phase as compared with the Maintenance Phase. No new participants in the Long-acting arm reported a drug-related adverse event during the Extension Phase. No serious adverse event was considered related of long-acting CAB+RPV treatment. During the Extension Phase, two participants in the Long-acting arm discontinued because of adverse events, which were identified as acute hepatitis B (Grade 3, considered not related to study treatment) and fear (Grade 1, considered related to study treatment). One participant in the Switch arm discontinued because of two adverse events of injection site pain. No deaths were reported during the Extension Phase in either treatment arm.
Injection site reactions
Among 2627 injections administered during the Extension Phase (Long-acting arm, n = 1363; Switch arm, n = 1264), 392 ISRs were recorded (Long-acting arm, n = 154; Switch arm, n = 238) (Table 2). Most ISRs were Grade 1 (mild) or Grade 2 (moderate) in severity [Long-acting arm, 100% (n = 154/154); Switch arm, 99% (n = 235/238)], except for three single Grade 3 adverse events of injection site pain in the Switch arm; none were considered serious by investigators. The most frequently occurring ISR event was injection site pain [Long-acting arm, 78% of ISR events (n = 120/154); Switch arm, 87% of ISR events (n = 207/238)]; all other ISRs had an incidence of less than 5% in each arm, with the exception of injection site induration [Long-acting arm, 5% of ISR events (n = 8/154); Switch arm, 6% of ISR events (n = 14/238)]. No participants in the Long-acting arm discontinued because of reasons related to injections during the Extension Phase. Two participants in the Switch arm withdrew for reasons related to injections: one because of two Grade 2 events of injection site pain and another because of intolerability of injections.
Laboratory evaluations and vital signs
The incidence values of clinical laboratory abnormalities in the Extension Phase are detailed in Supplemental Digital Content Table S2, https://links.lww.com/QAD/C245, which summarizes maximum emergent chemistry toxicities. Two participants in the Extension Phase (both arm) had alanine aminotransferase elevations to at least three times the upper limit of the normal range, both of which met protocol-defined liver-related stopping criteria. Both participants were found to have viral hepatitis; one participant tested positive for acute hepatitis E and the other participant had acute hepatitis B. In the Long-acting and Switch arms, median (IQR) bodyweight increased throughout the Extension Phase: a weight gain of 2.1 (-1.0, 5.0) kg from Baseline to W 96 occurred in the Long-acting arm (n = 23); a weight gain of 1.1 (0.0, 3.1) kg from W 52 to W 96 occurred in the Switch arm (n = 29). Similarly, increases in median (IQR) BMI were also recorded [Long-acting arm, 0.7 (-0.4, 1.5) kg/m2 increase from Baseline; Switch arm, 0.3 (0, 1.1) kg/m2 increase from Extension Baseline].
Pharmacokinetic analysis
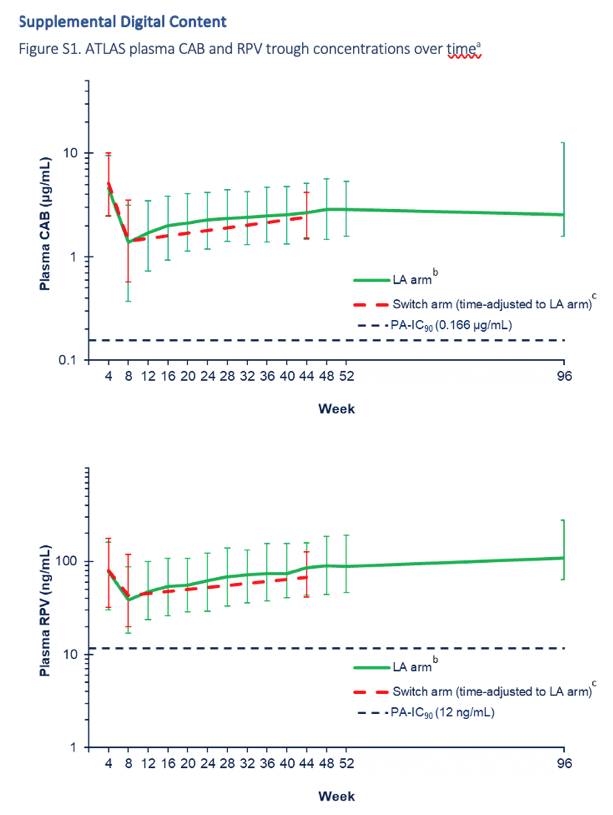
In both the Switch and Long-acting arms, median plasma CAB and RPV trough concentrations remained above their respective protein binding-adjusted 90% inhibitory concentration (PA-IC90: CAB, 0.166 μg/ml; RPV, 12.0 ng/ml) throughout the Extension Phase (Supplemental Digital Content Figure S1, https://links.lww.com/QAD/C245, which illustrates median plasma CAB and RPV trough concentrations collected throughout the study). For the Switch arm, median W 96 CAB and RPV trough concentrations after 40 weeks of intramuscular injections were 1.7-fold and 1.6-fold higher, respectively, than at W 60, 4 weeks following the initial intramuscular loading dose [CAB: W 60, 1.41 μg/ml (n = 127); W 96, 2.42 μg/ml (n = 24); RPV: W 60, 42.9 ng/ml (n = 127); W 96, 66.7 ng/ml (n = 24)]. For the Long-acting arm, the median W 96, CAB trough concentration after 92 weeks of intramuscular injections was comparable with that observed in the Switch arm [Long-acting arm, 2.56 μg/ml (n = 19); Switch arm, 2.42 μg/ml (n = 24)], consistent with an achievement of steady state after ∼44 weeks of injections. For RPV, the median W 96 plasma trough concentration in the Long-acting arm was higher than that in the Switch arm [Long-acting arm, 109.0 ng/ml (n = 19); Switch arm, 66.7 ng/ml (n = 24)]. This is consistent with limited further accumulation in the second year of injections, in line with the reported 28-week half-life of long-acting RPV [21].
|
|
| |
| |
|
|
|