 |
 |
 |
| |
Real-world utilization of F/TDF and F/TAF
for HIV Pre-exposure Prophylaxis during the
COVID-19 pandemic in the US, December 2019-June 2020
|
| |
| |
New US PrEP Use Dropped in Early COVID Months, Then Rebounded
IAS 2021, 11th IAS Conference on HIV Science, July 18-21, 2021
Mark Mascolini
Numbers of new preexposure prophylaxis (PrEP) users plunged in the United States in the first months of the COVID-19 pandemic, according to analysis of pharmacy claims representing at least 80% of US retail pharmacies [1]. The steep dip in new (incident) PrEP users starting in January 2020 rebounded slightly in late spring and summer but never returned to 2019 prepandemic levels. In contrast, the number of ongoing (prevalent) PrEP users dipped only slightly in March, April, and May 2020, then returned to prepandemic highs.
Both emtricitabine/tenofovir disoproxil fumarate (F/TDF) and emtricitabine/tenofovir alafenamide (F/TAF) are licensed for HIV prevention in at-risk US adults and adolescents. Generic F/TDF became available in October 2020 in the United States. The COVID-19 pandemic had profound impacts on healthcare and social behavior across the world, but its effect on PrEP adoption and continued use remained unknown until this analysis by researchers at Gilead Sciences, makers of F/TDF and F/TAF.
Gilead researchers set out to chart real-world US patterns in F/TDF and F/TAF PrEP from December 1, 2019 (just before the pandemic began) to December 31, 2020 (when the pandemic had waned and stabilized in the United States). The investigators gathered de-identified data on F/TDF and F/TAF prescriptions filled from a national electronic pharmacy claims database representing at least 80% of all US retail pharmacies. They used a validated algorithm to pinpoint PrEP users of F/TDF (branded and generic) and F/TAF by excluding use of the 2-in-1 pills for HIV treatment, postexposure prophylaxis (PEP), and off-label treatment of chronic hepatitis B.
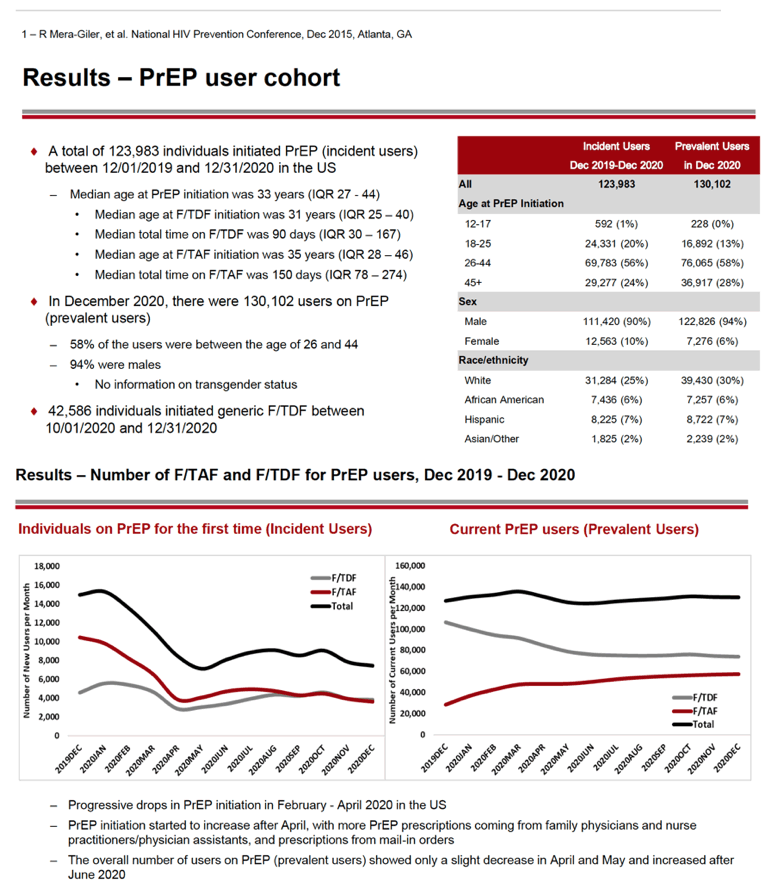
This analysis calculated that 123,983 individuals started PrEP (incident users) between December 1, 2019 and December 31, 2020 in the United States. When they started F/TDF or F/TAF, incident users had a median age of 33 years (interquartile range 27 to 44). Median total time using PrEP during the study window was 90 days for F/TDF and 150 days for F/TAF. From October 1 through December 31, 2020, 42,586 individuals started generic F/TDF.
In December 2020 the Gilead team counted 130,102 prevalent PrEP users. More than half of incident (56%) and prevalent (58%) PrEP users were between the ages of 26 and 44, while 24% and 28% were 45 or older. Men made up large majorities of prevalent PrEP users (94%) and incident PrEP users (90%). Pharmacy claims did not have information on race/ethnicity for about half of PrEP users. Among those with such data, most incident and prevalent users were white (25% and 30%), while much smaller proportions were black (6% and 6%), Hispanic (7% and 7%), or Asian/other (2% and 2%).
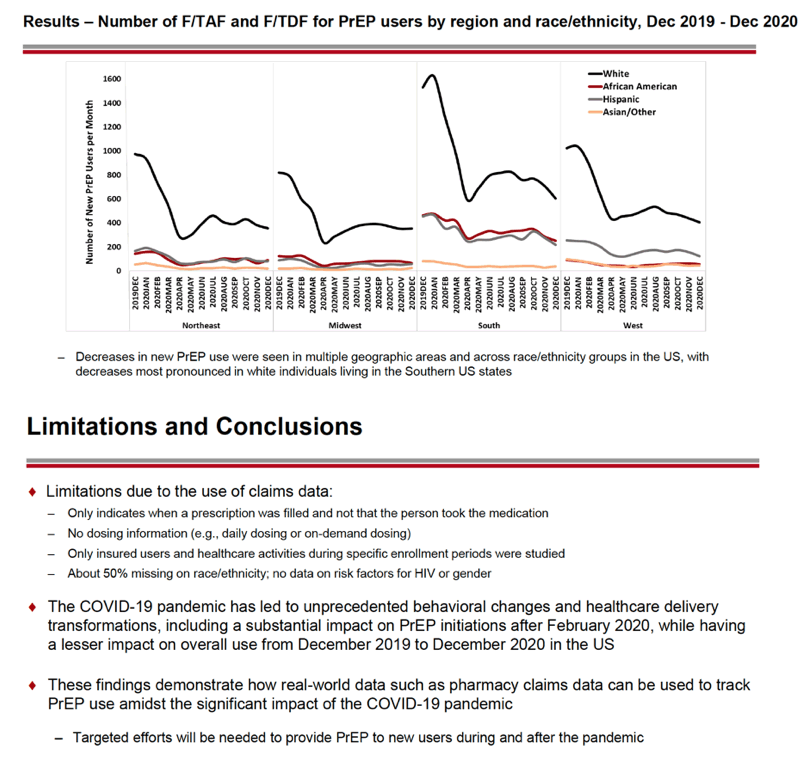
From December 2019, the number of first-time (incident) PrEP users fell from about 16,000 to about 8000 in May 2020. At that point the number of incident PrEP prescriptions rebounded to about 10,000 in July 2020 then changed little through December 2020. Incident PrEP use swooned in the first months of the pandemic in all four US regions analyzed-Northeast, Midwest, South, and West. Whites made up the biggest number of prepandemic incident PrEP users in all four regions, and whites had the steepest drops in new PrEP use. For example, the South had about 1600 new white PrEP users in January 2020, and that number nosedived to about 600 in April 2020 as the US pandemic reached its zenith. But incident PrEP use waned in all racial/ethnic groups when the pandemic struck.
The number of current (prevalent) PrEP users edged up from about 125,000 in December 2019 to about 140,000 in March 2020. Then prevalent PrEP prescriptions faded slightly to about 125,000 in May 2020 and stayed at about that level through December 2020. Over the whole study period, prevalent use of F/TDF declined slowly and steadily, while use of F/TAF rose steadily.
The Gilead investigators cautioned that these data indicate when a person filled a prescription, but not whether that person used the PrEP pills. Nor could the data distinguish between daily PrEP users and on-demand use (only before and after sex). The researchers believe their findings “suggest that COVID-19 has had a substantial impact on PrEP initiations, while having a lesser impact on overall use.”
Reference
1. Tao L, Carter C, Das M, Shvachko V, Magnuson D. Real-world utilization of F/TDF and F/TAF for HIV Pre-exposure Prophylaxis during the COVID-19 pandemic in the US, December 2019-June 2020. IAS 2021, 11th IAS Conference on HIV Science, July 18-21, 2021. Abstract OAC0203.

|
| |
|
 |
 |
|
|