 |
 |
 |
| |
Early treated individuals had lower reservoir size,
lower viral diversity and higher susceptibility to bNAbs
Evaluation of HIV-1 reservoir size and broadly neutralizing antibody (bNAb) susceptibility in individuals who initiated ART during acute and chronic infection
|
| |
| |
IAS 2021 July 18-22
Presenter: Brian Moldt
Authors: B. Moldt * (1 ), H. Gunthard (2), K. Workowski (3), S. Little (4), J. Eron (5), E. Overton (6), C. Lehmann (7), C. Rokx (8), M. Kozal (9), R. Gandhi (10), H. Liu (1 ), T. Makadzange (1 ), S. Collins (1 ), R. Geleziunas (1 ), C. Callebaut (1 ).
Institutions: (1) Gilead Sciences, Inc, Foster City, United States, (2) University Hospital Zurich, Zurich, Switzerland, (3) Emory University, Atlanta, United States, (4) University of California, San Diego, San Diego, United States, (5) University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, United States, (6) University of Alabama at Birmingham, Birmingham, United States, (7) University Hospital of Cologne, Cologne, Germany, (8) Erasmus University Medical Center, Rotterdam, Netherlands, (9) Yale School of Medicine, New Haven, United States, (10) Massachusetts General Hospital and Harvard Medical School, Boston, United States

Abstract:
BACKGROUND: Persistence of the viral reservoir is the main barrier to curing HIV. Initiation of ART during primary HIV infection can limit the size and diversity of the viral reservoir. Characterization of the differences between individuals who initiate ART during primary and chronic infection will be critical for clinical trial design and HIV cure strategies.
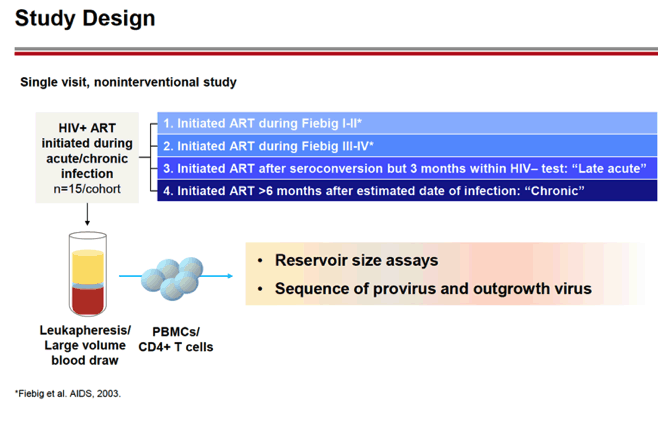
METHODS: A cross-sectional, non-interventional study was performed to characterize the viral reservoir in people living with HIV. Four cohorts were enrolled with participants that initiated ART during Fiebig 1-11, Fiebig Ill-IV, early (<=3 months of infection) or chronic (>=6 months of infection) infection. Participants underwent leukapheresis and viral reservoir in PBMCs was evaluated by the Intact Proviral DNA Assay (IPDA), the Total HIV DNA Assay (THDA), and the Quantitative Viral Outgrowth Assay (QVOA). Viral diversity and susceptibility to the bNAb elipovimab were determined by genotyping of the viral envelope gene.
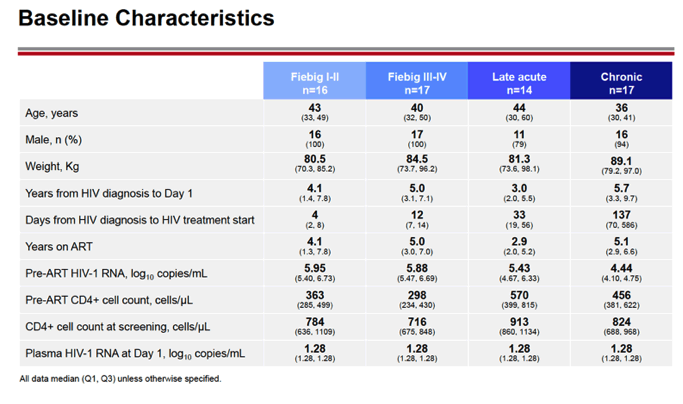
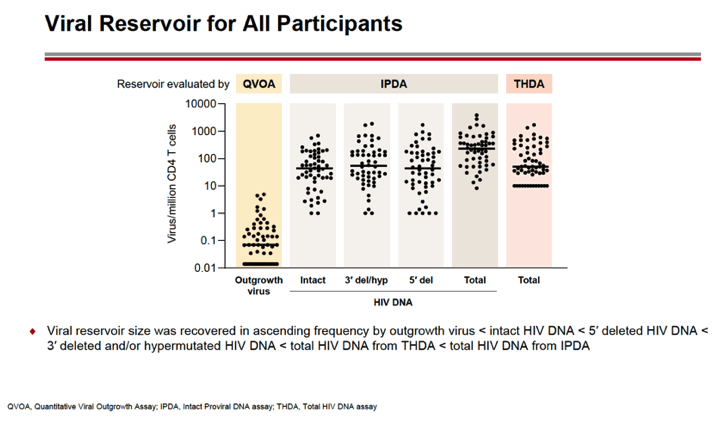
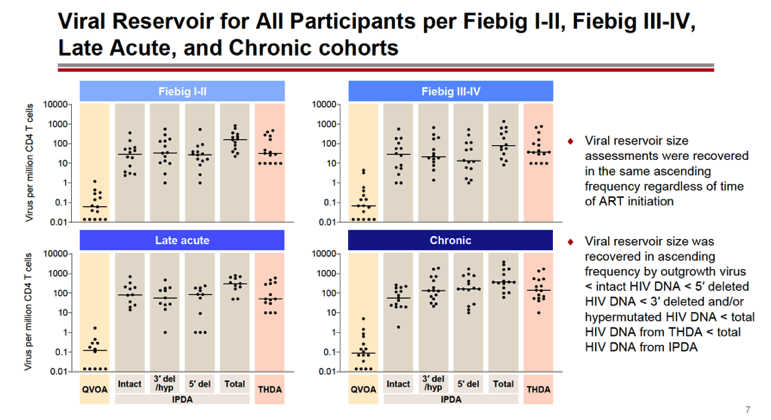
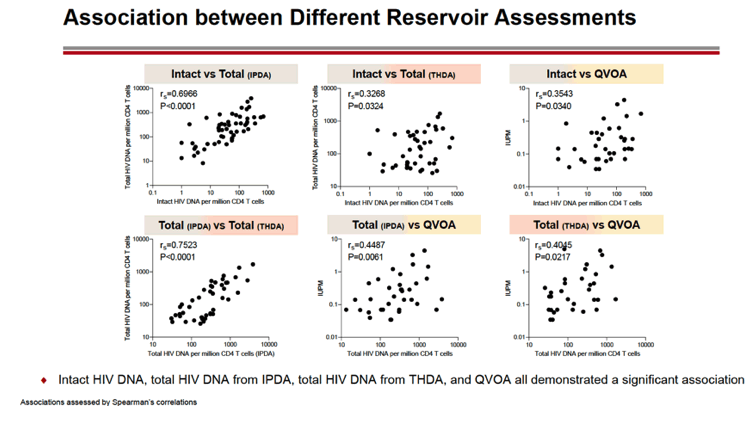
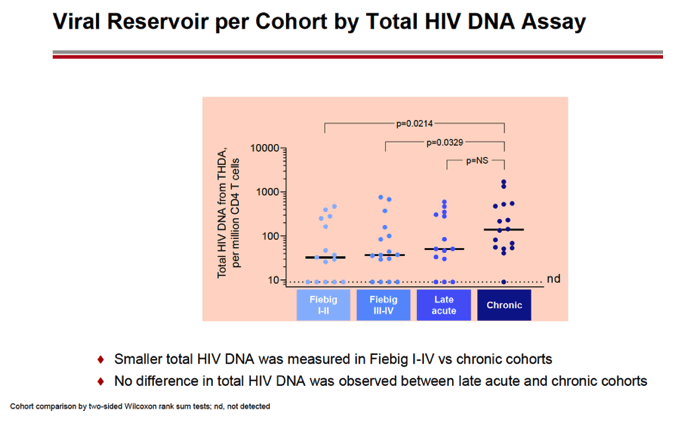
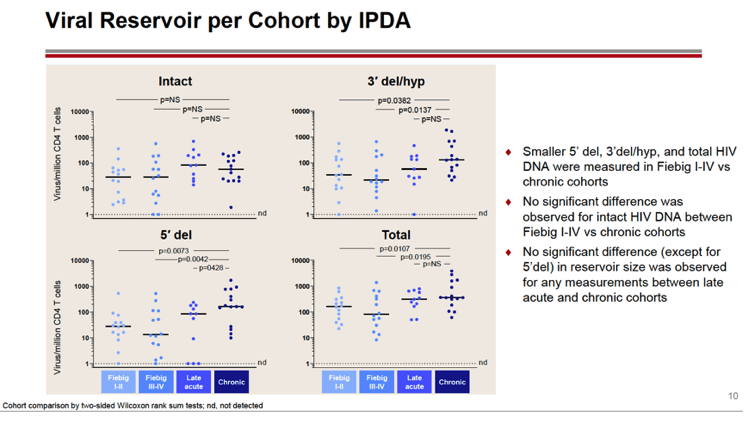
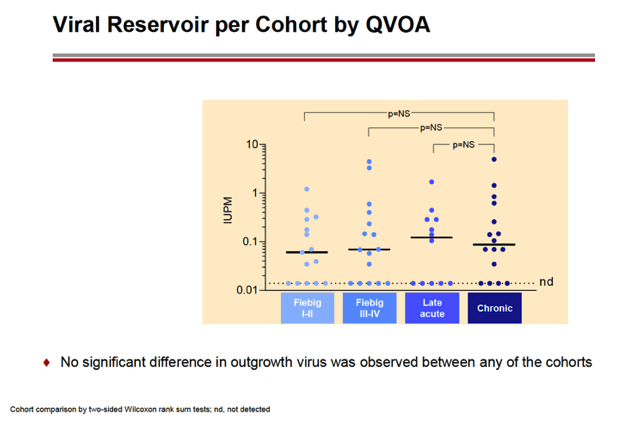
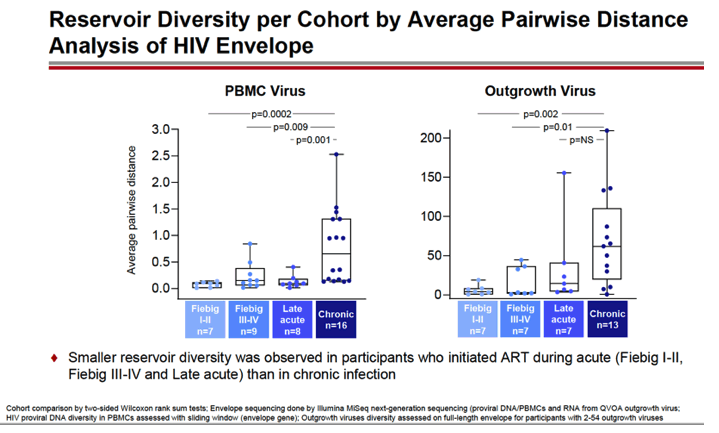
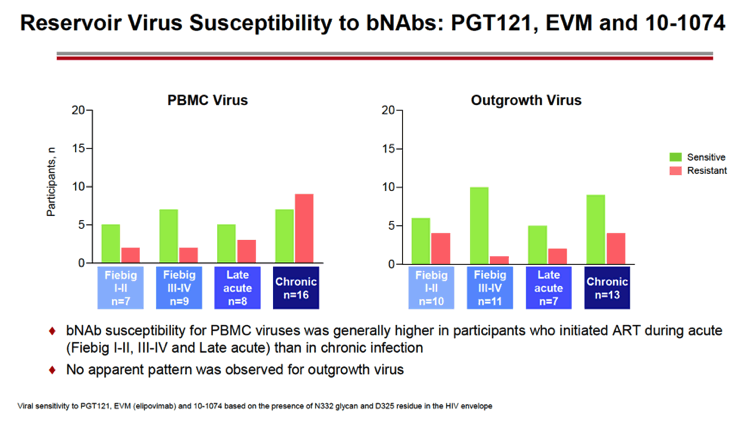
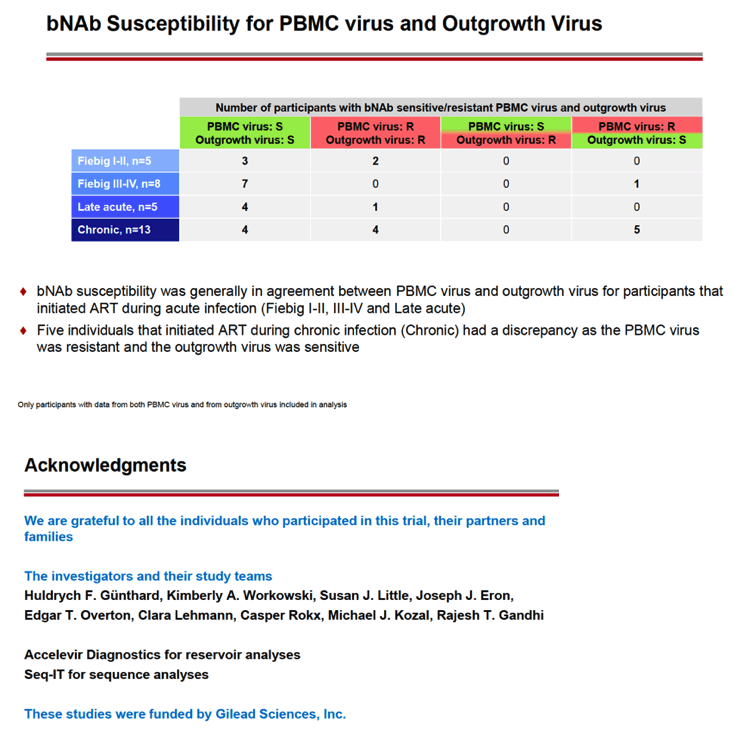
RESULTS: An increase in reservoir size was observed with increased time to ART initiation (Fiebig stages through chronic infection) when measured by IPDA and THDA whereas no difference was observed by QVOA. Viral diversity was lower in participants initiating ART during acute infection than chronic infection, and acute ART-treated individuals also showed higher susceptibility to elipovimab as 71 % of cohort 1 participants, 78% of cohort 2 participants, 53% of cohort 3 participants, and 44% of cohort 4 participants were sensitive.Table 1. Characteristic and HIV reservoir Cohort 1 Fiebig l-ll(n=16) Cohort 2Fiebig III-IV(n=17} Cohort 3 Early infection( n=14} Cohort 4 Chronic infection (n=17}Time on ART(years, median (01, 03)) 4.1 (1.3, 7 .8)5.0(3.0, 7.0) 2.9(2.0, 5.2) 5.1 (2.9, 6.6}Pre-ART HIV-1 RNA (log10 copies/ml, median (01, 03)) 5.95(5.40, 6.73)5.88(5.4 7, 6.69)5.43(4.67, 6.33) 4.44(4.10, 4.75)CD4+ cell count at screening(cellsli¼L, median (01, 03}} 784(636, 1109) 716(675, 848)913(860, 1134)824(688, 968) Intact HIV DNA, IPDA(copies/106 CD4+ cells, median (01, 03)) 28.86 (3.53, 58.09) 28.63 (24.67, 125.90)82.28 (14.07, 206.10)57.72 (20.42, 185.00) Cell-associated HIV DNA, IPDA (copies/106 CD4+ cells, median (01, 03)) 163.30 (50.47, 319.00) 79.92 (27.01, 465.40)308.50 (164.10, 641.30)359.50 (184.10, 1583.00) Cell-associated HIV DNA, THDA (copies/106 CD4+ cells, median (01, 03)) 32.40(9.00, 249.15) 37.12(19.18, 129.08) 50.77(29.95, 304.60) 138.27(54.31, 499.81} Replication competent HIV, QVOA(copies/106 CD4+ cells, median (01, 03)) ""0.060(0.014, 0.286) 0.069(0.014, 0.315) 0.105(0.014, 0.286) 0.070(0.014, 0.257)
CONCLUSIONS: Early treated individuals had lower reservoir size, lower viral diversity and higher susceptibility to bNAbs (exemplified by elipovimab) supporting that individuals who initiate ART during Fiebig stages, and in particular during Fiebig I to IV, would be an attractive population for early proof of concept bNAb cure-related trials. The IPDA provides both intact and total HIV DNA measurements and was able to differentiate between early and late cohorts and should therefore be given priority as a reservoir measurement in HIV cure trials.
|
| |
|
 |
 |
|
|