 |
 |
 |
| |
Risk of Virologic Failure Among Treatment-experienced Suppressed People with HIV (PWH) Treated with Single-Tablet 2-Drug (2DR) vs 3-Drug (3DR) Regimens
|
| |
| |
Doubled Risk of Virologic Failure With Switch to 2DR vs 3DR ART
IDWeek, September 29-October 3, 2021
Mark Mascolini
Switching to a single-tablet 2-drug antiretroviral regimen (2DR) rather than a single-tablet 3-drug regimen (3DR) doubled the risk of virologic failure in a 1668-person retrospective US study [1]. The finding runs counter to results of clinical trials of switching by people maintaining viral control with their current regimen.
Two single-tablet 2DR regimens-dolutegravir/rilpivirine (DTG/RPV) and DTG/lamivudine (3TC) have won approval as switch options for people whose current regimen has kept viral load undetectable. Randomized clinical trials found switching to licensed 2DRs can keep HIV under control in clinically stable people with no history of treatment failure. US researchers conducted this cohort study to see if 2DR and 3DR single-tablet regimens differ in maintaining viral suppression after a switch in clinical practice.
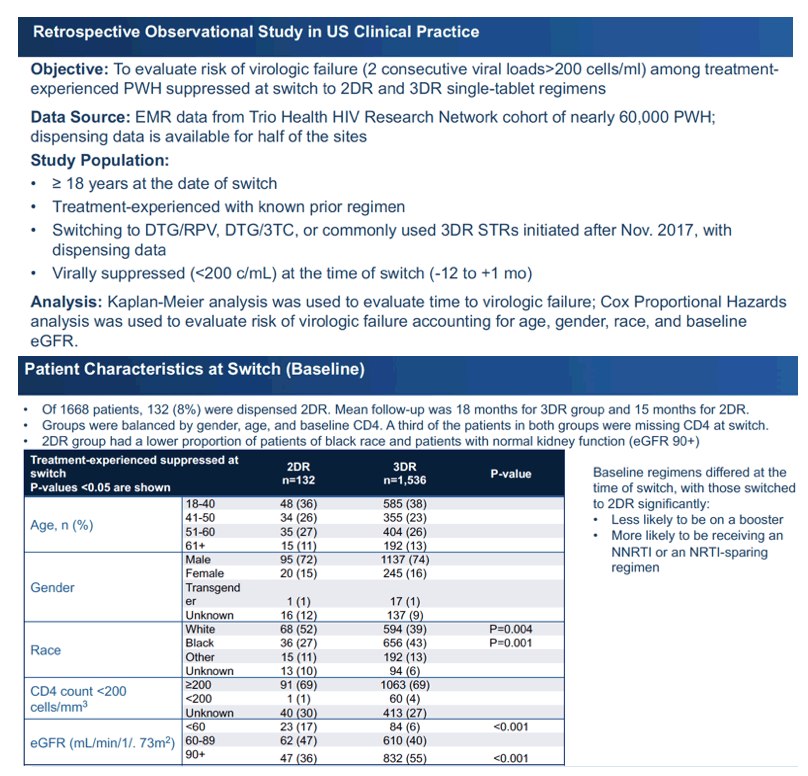
The researchers used data from the Trio Health HIV Research Network cohort, which includes almost 60,000 people with HIV [2]. Half of the study sites have antiretroviral dispensing data available. To participate in this analysis, cohort members had to be 18 or older and antiretroviral experienced with a known regimen. They needed a viral load below 200 copies when switching to DTG/RPV, DTG/3TC, or a common 3DR single-tablet regimen after November 2017. The researchers used Kaplan-Meier analysis to estimate time to virologic failure (2 consecutive viral loads above 200 copies). To evaluate risk of virologic failure, they used Cox proportional hazards analysis adjusted for age, gender, race, and baseline estimated glomerular filtration rate (eGFR)
The analysis focused on 132 adults switching to single-tablet 2DR and 1536 switching to single-tablet 3DR. Similar proportions in the 2DR and 3DR groups were 51 years or older (38% 2DR and 39% 3DR), about three quarters of each group were men, and 69% in each group had a CD4 count above 200. But almost one third of each group had no CD4-count recorded at the switch.
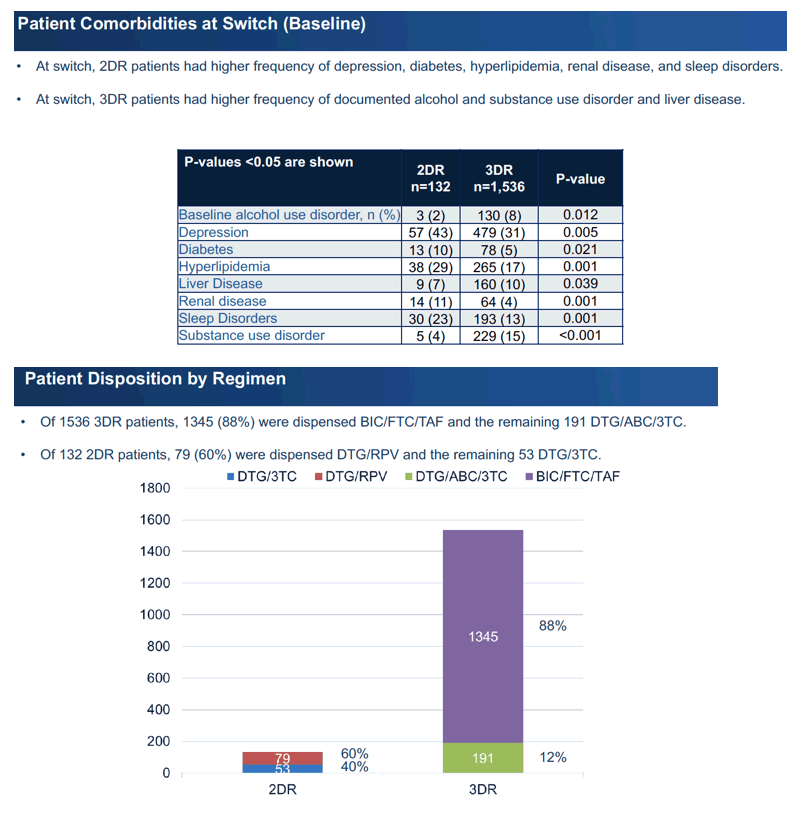
The 2DR group included a bigger proportion of whites than the 3DR group (52% vs 39%, P = 0.004) and a smaller proportion of blacks (27% vs 43%, P = 0.001). A lower proportion of the 2DR group had an eGFR above 90 mL/min, indicating healthy kidneys (36% vs 55%, P < 0.001). At the switch the 2DR group had higher proportions with depression, diabetes, hyperlipidemia, kidney disease, and sleep disorders and lower proportions with alcohol or substance use or liver disease.
Most 2DR participants (60%) took DTG/RPV, while the rest took DTG/3TC. Most 3DR participants (88%) took bictegravir/emtricitabine/tenofovir alafenamide, while the rest took DTG/abacavir/3TC. Follow-up averaged 15 months with 2DR and 18 months with 3DR.
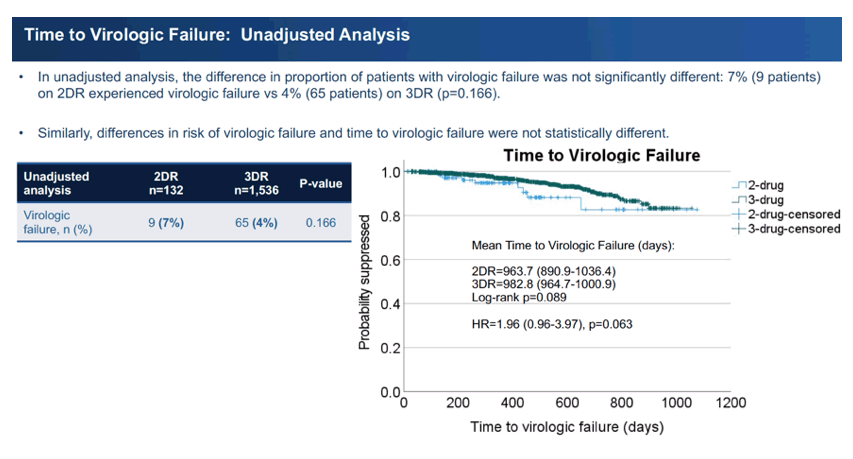
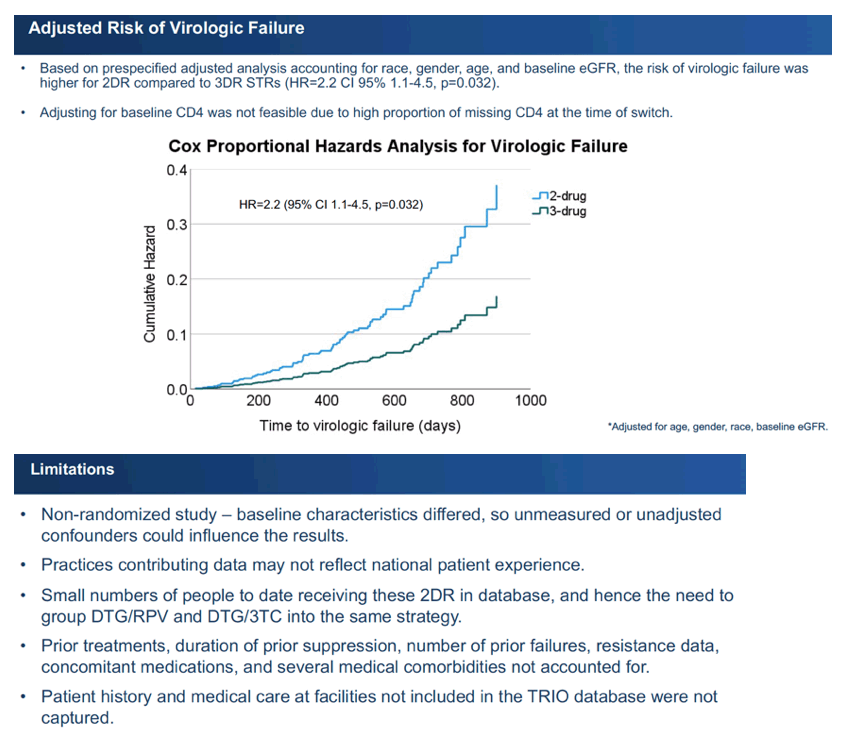
Unadjusted analysis determined that 7% (9 people) taking 2DR and 4% (65 people) taking 3DR had virologic failure, a nonsignificant difference (P = 0.166). Average time to virologic failure proved shorter in the 2DR group (963.7 versus 982.8 days), and this difference began to approach statistical significance (P = 0.089). A Cox proportional hazards model adjusted for age, race, gender, and eGFR at the switch determined that people switching to 2DR had a doubled risk of virologic failure compared with the 3DR group (adjusted hazard ratio 2.2, 95% confidence interval 1.1 to 4.5, P = 0.032). The analysis could not adjust for CD4 count because one third of each group did not have CD4 data at the switch.
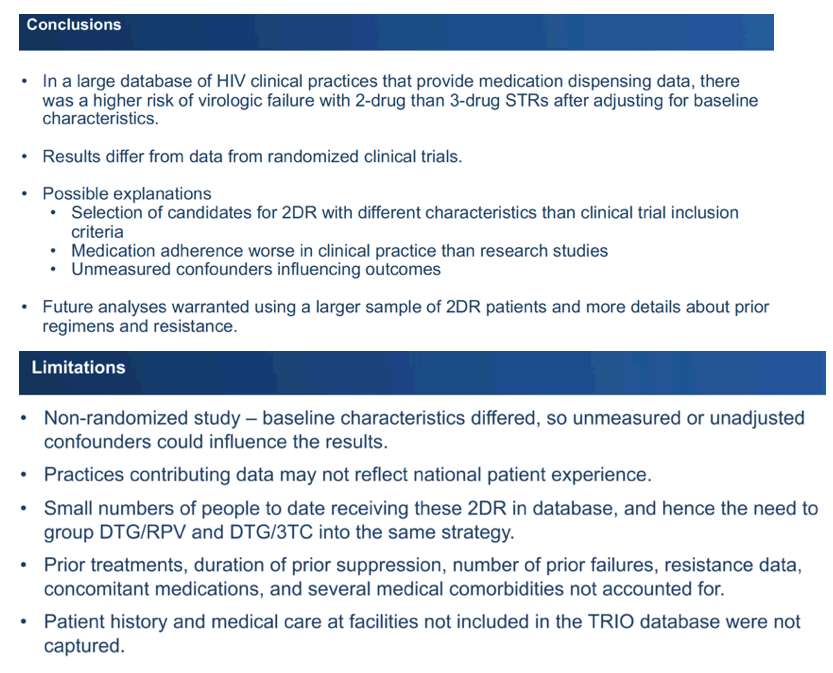
The researchers pointed out several limitations of their analysis: (1) It was a nonrandomized study with baseline differences between group, so confounders could have affected results. (2) Clinical practices contributing data may not reflect HIV practices across the United States. (3) The 2DR population was much smaller than the 3DR group. (4) The analysis did not account for prior antiretroviral regimens, number of regimen failures, resistance data, length of prior viral suppression, concomitant medications, or several comorbidities.
The investigators stressed that results differ from findings in randomized trials. That difference, they surmised, could reflect selection of 2DR candidates with different characteristics in this retrospective cohort versus clinical trials, worse antiretroviral adherence in clinical practice than in trials, and unmeasured confounders that could affect results. They called for further study of this issue with a bigger group of 2DR takers and more details about previous regimens and resistance.
References
1. Sax PE, Eron JJ, Radichenko J, et al. Risk of virologic failure among treatment-experienced suppressed people with HIV (PWH) treated with single-tablet 2-drug (2DR) vs 3-drug (3DR) regimens. IDWeek, September 29-October 3, 2021. Abstract 77.
2. Trio Health. Real Patient Insights. https://www.triohealth.com/overview


|
| |
|
 |
 |
|
|