 |
 |
 |
| |
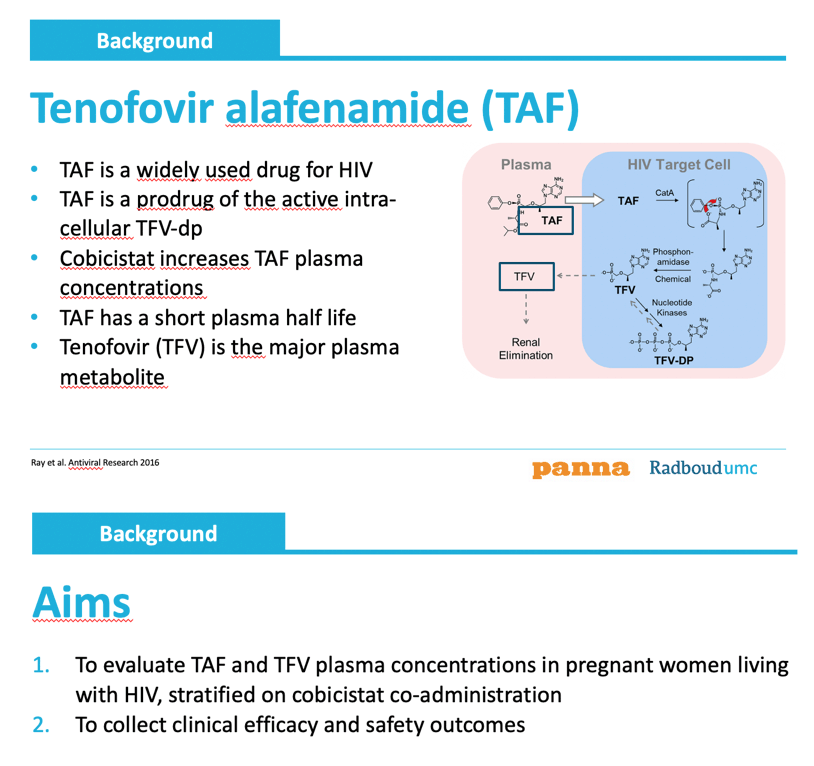
Tenofovir alafenamide concentrations are reduced
by half in pregnant women living with HIV: data from the PANNA Network.
|
| |
| |
TAF Levels About 60% Lower During Pregnancy But Still Effective
International Workshop on Clinical Pharmacology of HIV, Hepatitis and Other Antiviral Drugs, September 20-22, 2021
By Mark Mascolini for NATAP and Virology Education
Concentrations of tenofovir alafenamide (TAF) in plasma lay about 60% lower during the third trimester of pregnancy than after delivery, according to data from 20 women in the European PANNA study [1]. But the TAF-containing regimens remained clinically effective. The study presented by researchers at Nijmegen's Radboud University did not measure TAF in cells.
Understanding how drug concentrations may change during pregnancy is vital because physiological changes in pregnant women may affect drug levels. Although many preferred antiretroviral regimens include TAF, little is known about how pregnancy affects TAF exposure.
To address that question, Radboud University researchers and PANNA colleagues at other centers conducted this nonrandomized, open-label, multicenter study. PANNA is an ongoing study that prospectively collects pharmacokinetic data on newly developed antiretrovirals in pregnant women [2]. This analysis included pregnant women across Europe taking a TAF regimen. Researchers performed intensive pharmacokinetic sampling over 24 hours in the third trimester (about gestation week 33) and postpartum (about week 4 to 6). A central lab measured TAF plasma concentrations.
Among the 20 women studied, 80% were black, median age was 33 years, 50% took rilpivirine/emtricitabine/TAF 25/200/25 mg, and 30% took elvitegravir/cobicistat/emtricitabine/TAF 150/150/200/10 mg. Seven women (35%) took 10 mg of TAF daily with cobicistat, and the rest took 25 mg daily without cobicistat.
In the third trimester TAF geometric mean (GM) area under the concentration-time curve (AUC) in women also taking cobicistat stood at 99 ng*h/mL (95% confidence interval [CI] 61 to 161) and TAF GM maximum concentration (Cmax) measured 111 ng/mL (95% CI 55 to 226). In contrast postpartum TAF GM AUC was 270 ng*h/mL (95% CI 115 to 634) and GM Cmax 261 ng/mL (95% CI 67 to 1024). For women taking TAF without cobicistat, GM AUC was 97 ng*h/mL (95% CI 72 to 130) in the third trimester and 189 ng*h/mL (95% CI 120 to 298) postpartum. Respective GM Cmax were 83 ng/mL (95% CI 60 to 115) and 195 ng/mL (95% CI 131 to 292).
Among women taking TAF with cobicistat, third trimester/postpartum geometric mean ratios (GMR) of 0.37 (90% CI 0.20 to 0.69) for AUC and 0.42 (90% CI 0.27 to 0.66) for Cmax indicated that TAF concentration lay about 60% lower during the third trimester than after delivery. Among women taking TAF without cobicistat, third trimester/postpartum GMRs were 0.59 (90% CI 0.45 to 0.78) for AUC and 0.46 (90% CI 0.32 to 0.66) for Cmax.
Exposure of tenofovir (TFV), TAF's major metabolite, was 30% lower during the third trimester than postpartum: third trimester/postpartum GMRs were 0.67 (90% CI 0.62 to 0.74) for AUC, 0.70 (90% CI 0.62 to 0.80) for Cmax, and 0.66 (90% CI 0.60 to 0.71) for Ctrough.
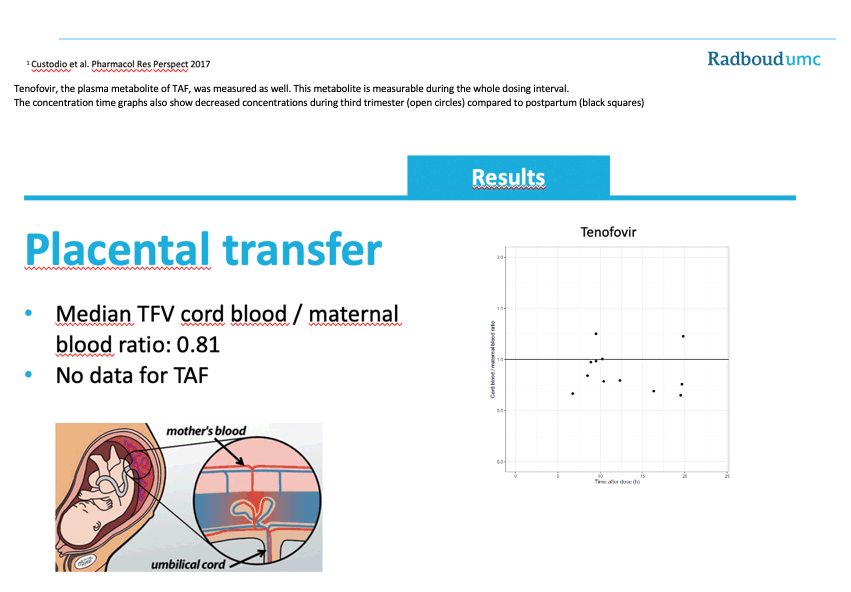
A median TFV cord blood/maternal blood ratio of 0.81 indicated some placental transfer of TFV.
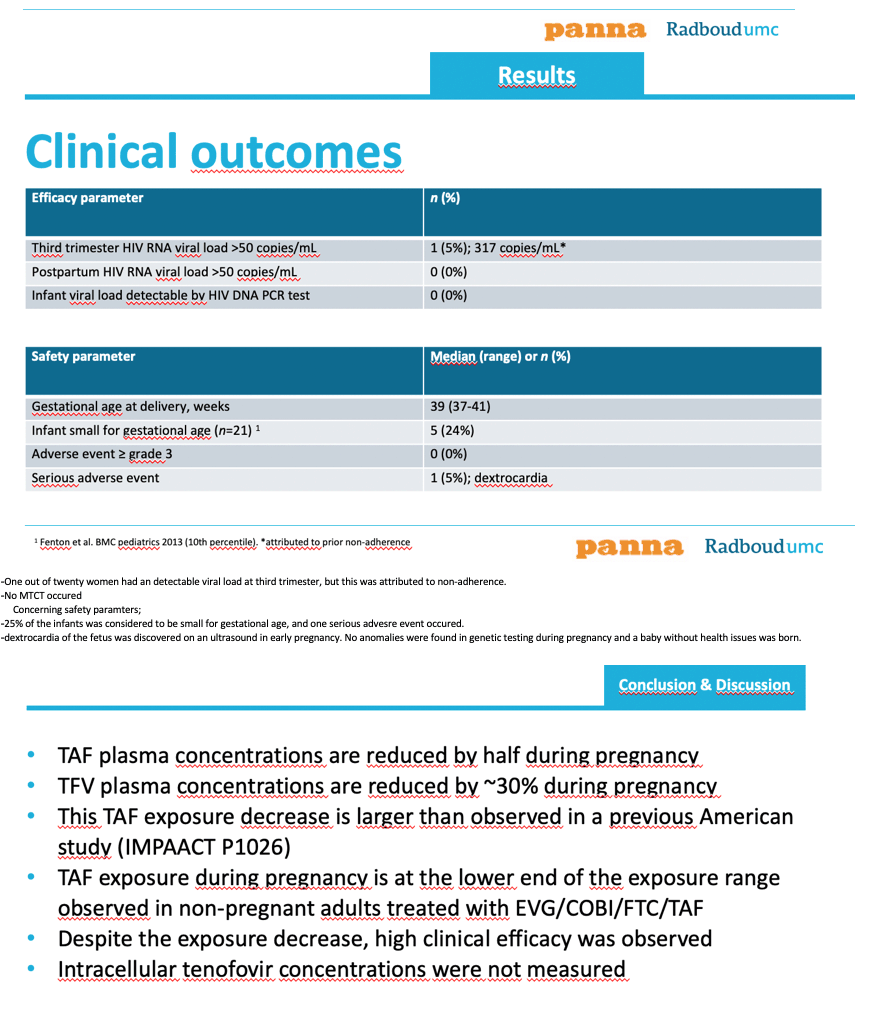
Only 1 of the 20 women had a detectable viral load (317 copies) during the third trimester, a finding attributed to wavering treatment adherence. All 20 women had an undetectable viral load at delivery and postpartum, and all newborns had a negative HIV DNA PCR at birth. Median gestational age at delivery stood at 39 weeks (range 37 to 41), and researchers observed no congenital abnormalities or grade 3 or 4 adverse events. They reported 1 serious adverse event (5%), dextrocardia. Five of 21 infants (24%) were small for their gestational age.
The research team noted that they recorded lower TAF levels during pregnancy than a previous US study of 58 pregnant women with HIV [3], possibly because of more intensive plasma sampling in the PANNA study. They added that their observed TAF AUC during pregnancy lay at the lower end of the range seen in nonpregnant adults taking elvitegravir/cobicistat/emtricitabine/TAF 150/150/200/10mg (mean 210 ng*h/mL, standard deviation 150) [4].
References
1. Bukkems V, Necsoi C, Hidalgo-Tenorio C, et al. Tenofovir alafenamide concentrations are reduced by half in pregnant women living with HIV: data from the PANNA Network. International Workshop on Clinical Pharmacology of HIV, Hepatitis and Other Antiviral Drugs 2021. September 20-22, 2021. Abstract 11.
2. PANNA. https://www.pannastudy.com/
3. Brooks KM, Momper JD, Pinilla M, et al, IMPAACT P1026s Protocol Team. Pharmacokinetics of tenofovir alafenamide with and without cobicistat in pregnant and postpartum women living with HIV. AIDS. 2021;35:407-417. doi: 10.1097/QAD.0000000000002767. https://pubmed.ncbi.nlm.nih.gov/33252495/
4. Genvoya Product Information. https://www.genvoya.com

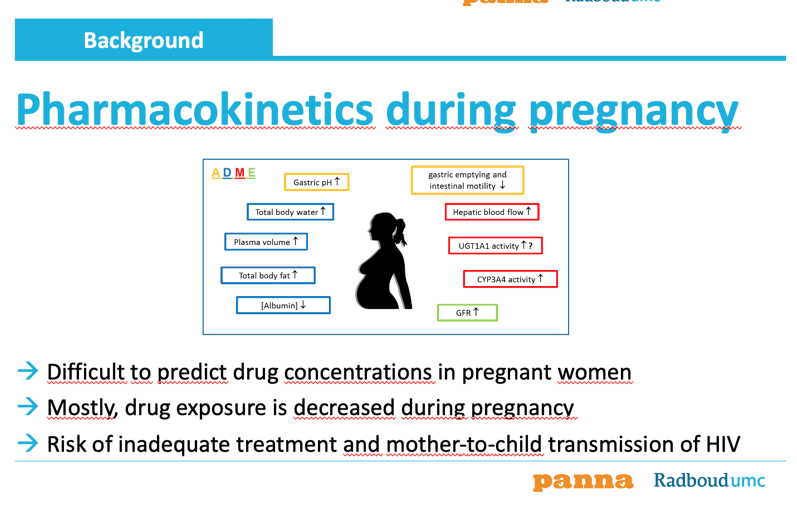
Those of you, who have been pregnant, know that your body completely changes during pregnancy. These physiological changes influence the absorption, distribution, metabolism and elimination of drugs. As depicted on the slide, a variety of physiological processes changes making it difficult to predict the resulting drug exposure in pregnant women. From previous research we know that the drug exposure is generally decreased in pregnant women. Decreased drug concentrations means the persons is at risk for inefficacious treatment. Also, adeqaute treatment is of particulaor importance in pregnant women to prevent mother-to-child-transmission of HIV. This prevent infecting the baby with a live-long chronic disease.
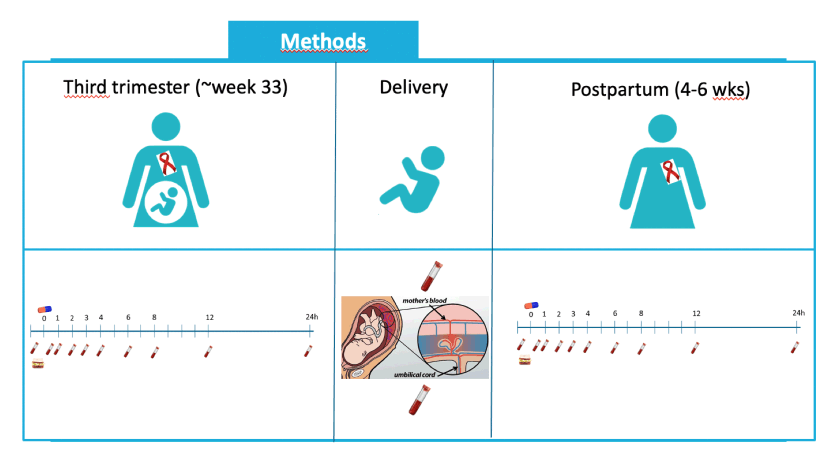
-Pregnant women living with HIV treated with a TAF-containing regimen were included from HIV-treatment centers across Europe.
-Intensive pharmacokinetic sampling over 24 hours (according to the scheme depticted on the slide) was performed in the third trimester (∼week 33) and postpartum (∼4-6 weeks).
-At delivery a cord blood and mother blood sample was taken to evaluate placental transfer
-The collected plasma samples were centrally analyzed with a validated LC-MS/MS assay
-TAF pharmacokinetic parameters were subsequently determined with non-compartmental analysis
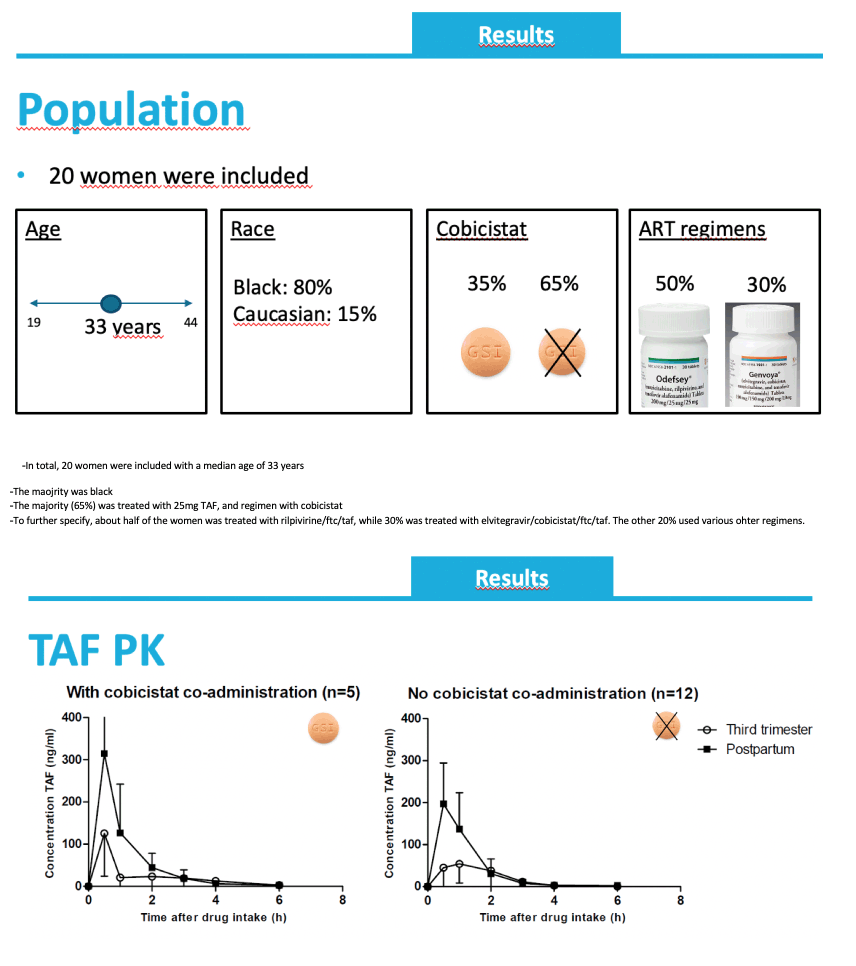
These are the pharmacokinetic resutls.
The mean concentration time profiles are shown over here, on the left for women treated with a cobicistat-including regimen on the right for women using a regimen without cobicistat. TAF plasma concentrations were quantifiable untill 6hours after drug intake. At third trimester (open circles) lower TAF concentrations were observed as during postpartum (black squares).
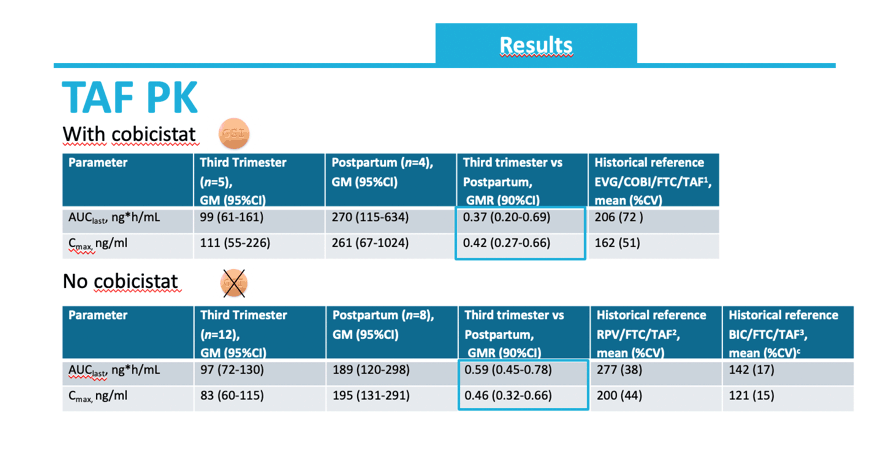
-Next the pharmacokinetic parameters were determinder, first shown for the group with cobicistat
-The geometric mean AUClast was 99 during pregnancy, and the Cmax 111 ng/ml
-These corresponded to a 63% decrease in AUC during pregnancy and a 58% decrease compared to postpartum
For women using a regimen without cobicistat, similar exposures were observed during pregnancy. A 41% decrease in AUC and 54% decrease in Cmax was observed during pregnancy.
Extra:
- BIC PK parameters waren niet per se lager (te weinig data)
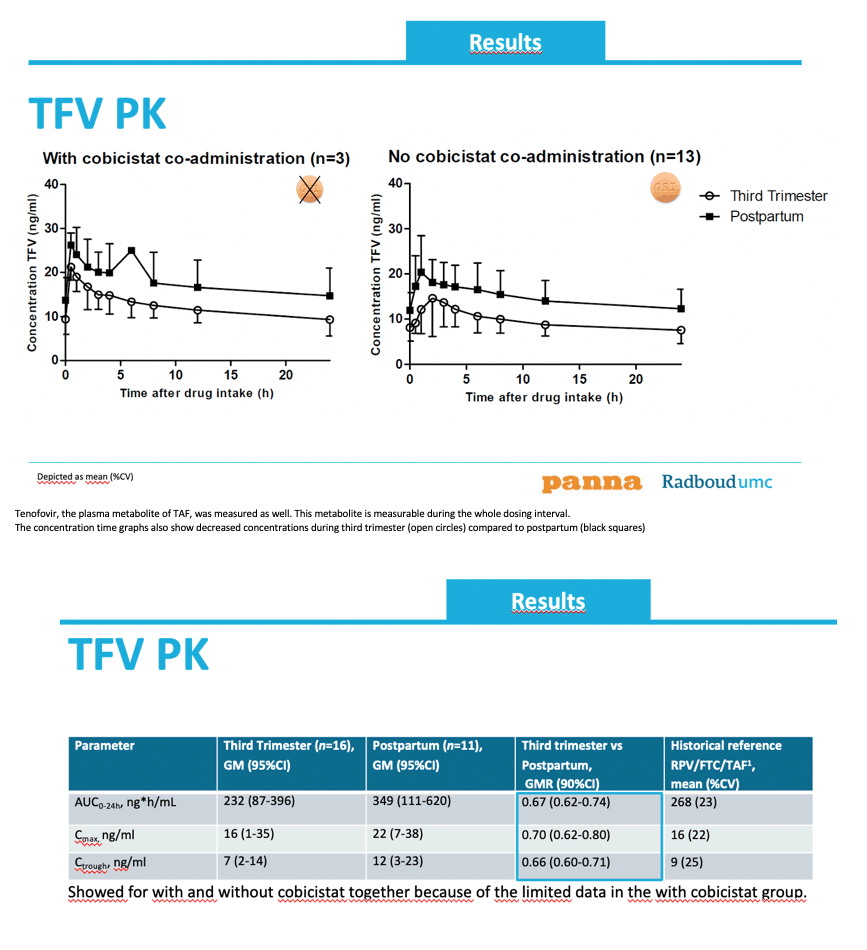
Tenofovir, the plasma metabolite of TAF, was measured as well. This metabolite is measurable during the whole dosing interval.
The concentration time graphs also show decreased concentrations during third trimester (open circles) compared to postpartum (black squares)

|
| |
|
 |
 |
|
|