 |
 |
 |
| |
Under Two Thirds With Viremic HCV and Insurance Get DAAs in US
|
| |
| |
AASLD-The Liver Meeting, November 4-8, 2022, Washington, DC
Mark Mascolini
Under two thirds of insured US residents with detectable HCV RNA got direct-acting antiviral (DAA) therapy in 2014-2021, according to analysis of an insurance claims database [1]. Only a little more than half of people with hepatic decompensation or hepatocellular carcinoma (HCC) got DAAs.
Although DAAs rank among the most-studied drugs in the history of pharmacotherapy, researchers at Stanford University and colleagues at other centers noted that real-world nationwide estimates of DAA use are scarce. So they decided to explore DAA prescribing in the United States since these agents became available, including their use in the COVID era.
The investigators tapped the OPTUM Clinformatics insurance claims database for 2014-2021 to determine DAA use rates, using adjusted log-binomial regression to figure changes over time. They relied on multivariable logistic regression to identify factors independently associated with DAA therapy.
The researchers found 133,348 people with HCV, including 38,180 with HCV RNA data and 20,277 with a positive HCV RNA result. The study group averaged 59.7 years in age, 56.1% were white, 59.7% male, and 50.2% college graduates. About 1 in 5 people in the study cohort had cirrhosis or HCC or both.
Just under two thirds of people positive for HCV RNA, 65%, got DAA therapy, and 97% of them had a sustained virologic response (SVR). A higher proportion of the subset with compensated cirrhosis, 76.5%, received DAA therapy, but only 56% of those with hepatic decompensation or HCC and 63% of people without cirrhosis got treated.
Among HCV-RNA positive people cared for by gastroenterologist or infectious disease (GI/ID) specialists with advanced practice providers (APP), nearly 75% had DAA therapy, compared with 50.4% of people seen by primary care physicians without APP (P < 0.001).
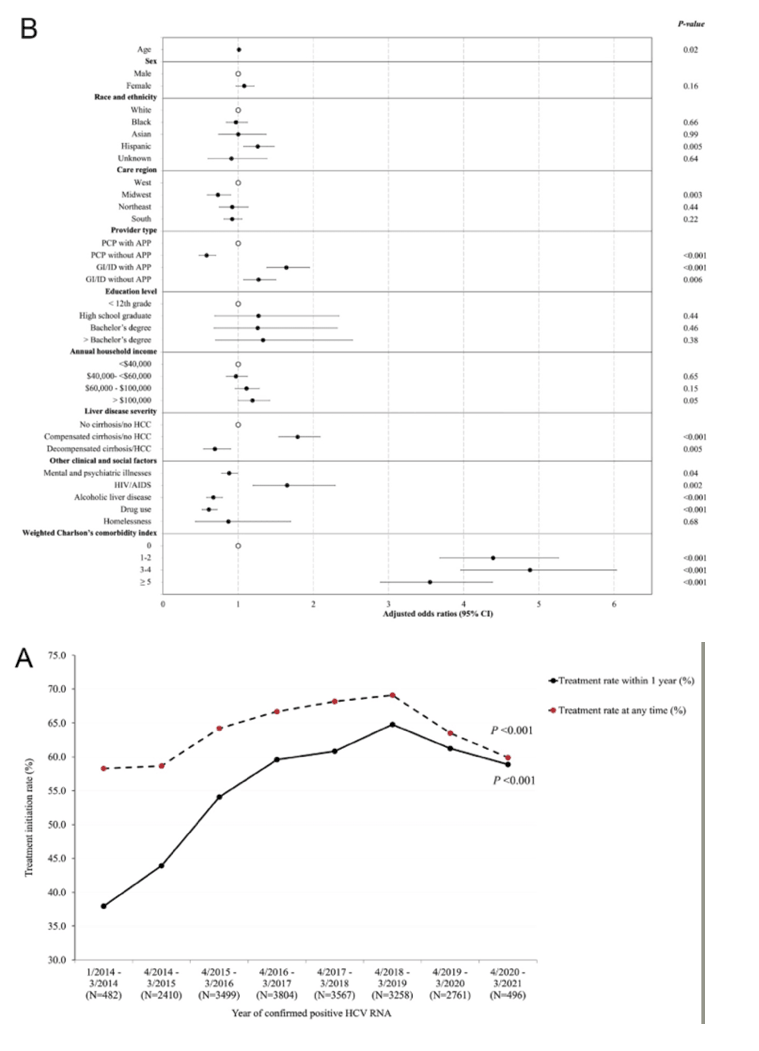
An analysis adjusted for age, sex, and race/ethnicity determined that the DAA treatment rate in 2014 rose 50% to 2018 (adjusted prevalence ratio 1.50, 95% confidence interval [CI] 1.42 to 1.59). But the treatment pace started slipping after 2018, and that decline steepened after 2019, when COVID arrived in the United States (P < 0.001). This falling treatment rate reflects a plunge in people positive for HCV RNA to 496 in the period April 2020-March 2021, down from 2761 viremic people the year before and from 3258 a year before that.
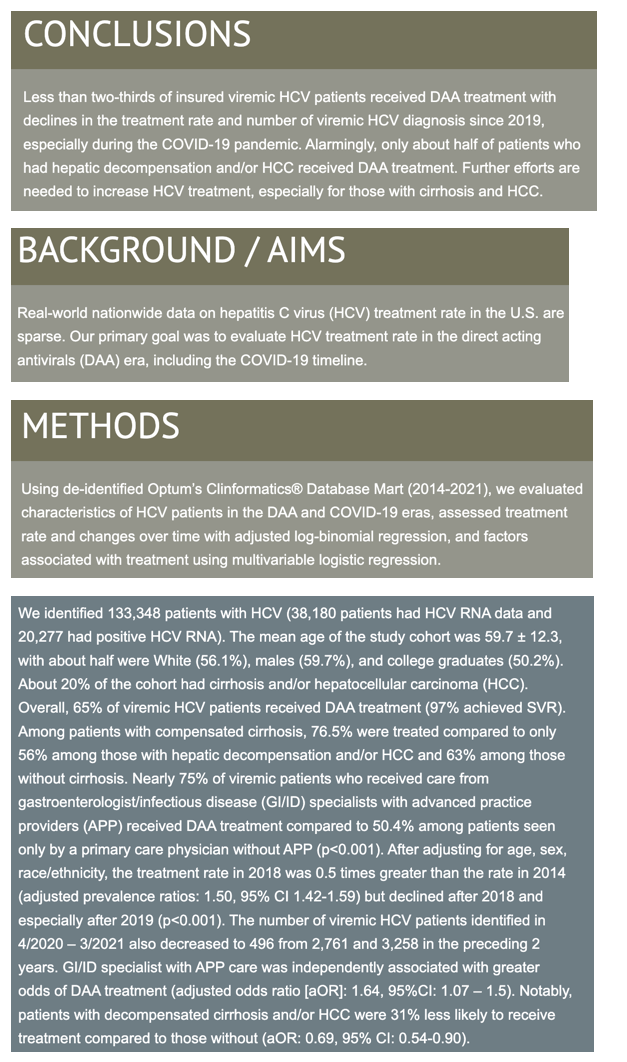
Multivariable logistic regression linked receiving care from GI/ID specialists with APP versus primary care clinicians to two thirds higher odds of getting DAA therapy (adjusted odds ratio [aOR] 1.64, 95% CI 1.07 to 1.5).People with decompensated cirrhosis or HCC had 31% lower odds of benefitting from DAAs than people without those conditions (aOR 0.69, 95% CI 0.54 to 0.90).
Other variables independently associated with higher odds of DAA therapy were being Hispanic (versus white), having an annual household income above $100,000 (versus under $40,000), having compensated cirrhosis without HCC (versus no cirrhosis and no HCC), having HIV infection, and having a higher weighted Charlson comorbidity index (versus no comorbidities). Other factors independently associated with lower odds of DAA therapy were living in the Midwest (versus the West) and having a mental or psychiatric illness, having alcoholic liver disease, or using drugs. Education level did not affect odds of getting DAAs in this analysis.
The researchers call low treatment rates of people with hepatic decompensation or HCC alarming. They endorse further efforts to raise rates of DAA therapy, especially for people with cirrhosis and HCC.
Reference
1. Nguyen VH, Kam L, Yeo YH, et al. Characteristics and treatment rate of patients with hepatitis C virus infection in the DAA and COVID-19 eras in the United States. Abstract 1278.
|
| |
|
 |
 |
|
|