 |
 |
 |
| |
Hormonal Intervention Combined with Allopregnanolone Alleviates Premature Age-Related Comorbidities in Female Mice Exposed to Ovarian Failure and HIV-1 Tat
|
| |
| |
Aging Workshop Oct 14-15 2022
Qrareya A1, Mahdi1 F1, Ashpole N1,2, Paris J1,2
1The University Of Mississippi, Lafayette Springs, United States, 2Research Institute of Pharmaceutical Science, University, United States
Background: In the U.S, over 50% of people living with HIV (PLWH) are aged 50 or older. However, age-related comorbidities occur frequently among PLWH compared to age-matched seronegative people. Women with HIV experience an earlier transition to post-menopause that is associated with a higher incidence of age-related comorbidities. Despite antiretroviral therapy, neurotoxic HIV proteins such as the trans-activator of transcription (Tat) persist within the central nervous system and may contribute to age-related comorbidities. We find that Tat is sufficient to dysregulate aspects of the neuroendocrine system in mice. However, the benefit of hormonal replacement therapy (HT) in HIV is unclear.
Material and Methods: We use 4-vinylcyclohexene diepoxide to achieve an ovary-intact peri- and post-menopause model in Tat-transgenic mice. We hypothesized that conditional Tat expression [Tat(+)] would exacerbate age-related comorbidities compared to age-matched controls [Tat(-)]. We anticipated HT would attenuate Tat/age-related comorbidities to a greater degree when intervention occurred in the peri-estropausal stage, as opposed to the post-estropausal stage. We also assessed the effects of the most commonly-prescribed HT, Prempro® (conjugated equine estrogens and medroxyprogesterone acetate), alone or in combination with a neuroprotective progesterone metabolite (allopregnanolone; AlloP). Behavioral data were analyzed using three-way ANOVA.
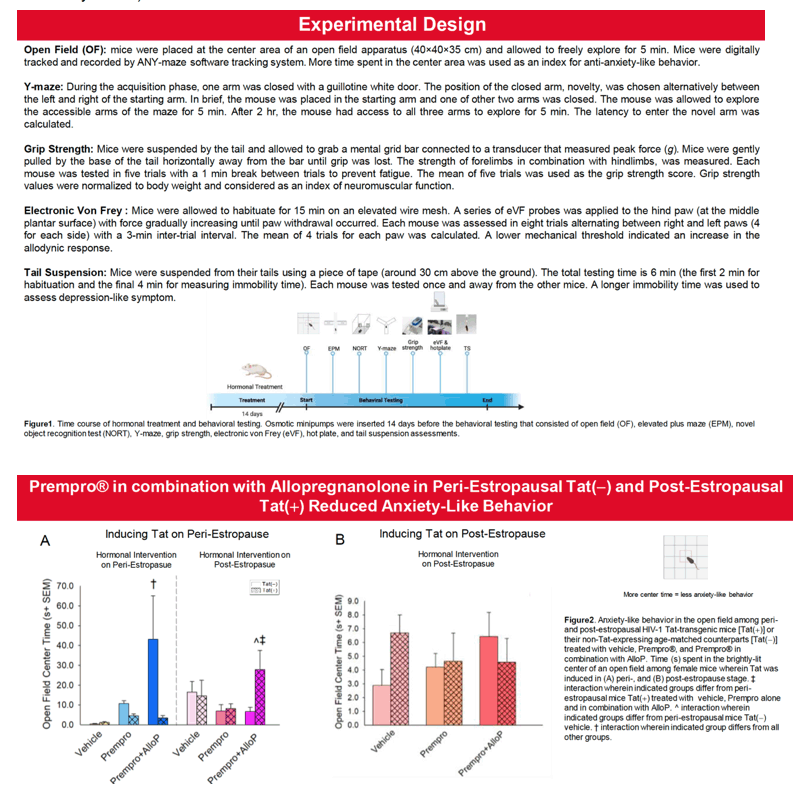
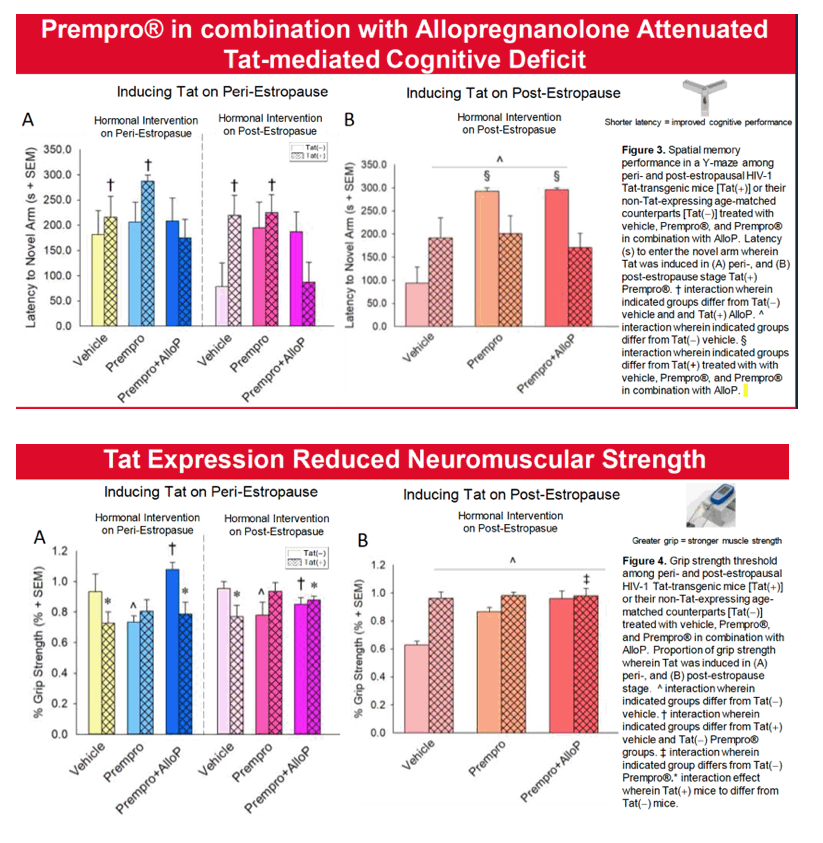
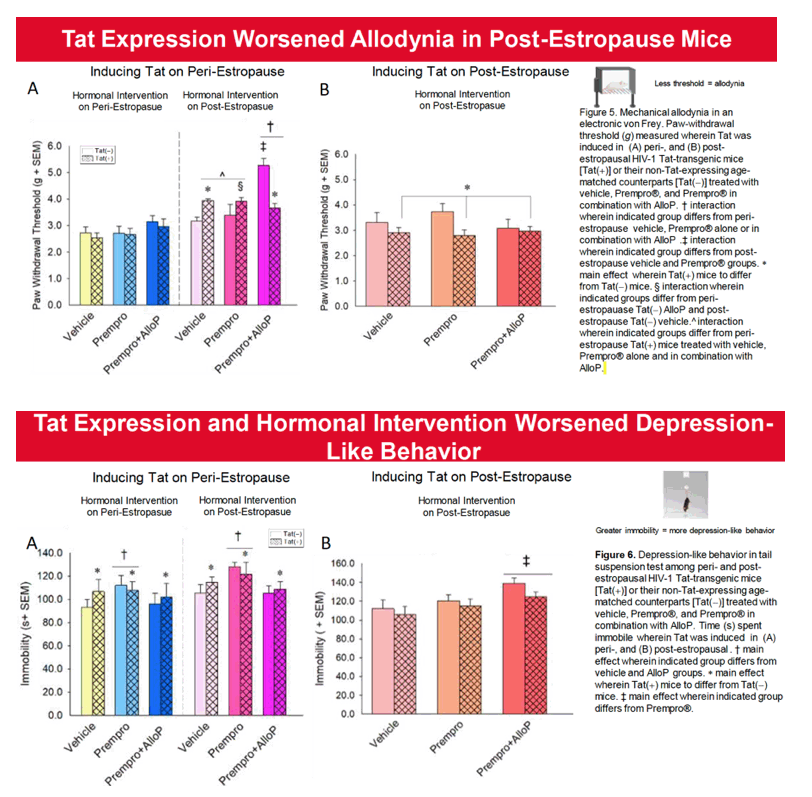
Results: Overall, post-estropausal mice demonstrated greater anxiety- and depression-like behavior than did peri-estropausal mice. When exposure to Tat occurred in the peri-estropausal stage, mice exhibited little initial change in nociceptive sensitivity or neuromuscular function; however, as Tat exposure continued throughout the post-estropausal transition, mechanical nociceptive thresholds were increased. Neuromuscular function generally declined. These findings are consistent with a reduction in mechano-sensitivity and muscle strength as exposure to Tat becomes chronic. Notably, if Tat exposure first occurred in the post-estropausal stage, mechanical pain responses were much greater. The anti-anxiety-like benefits of Prempro® were only observed when HT was initiated in the peri-estropausal stage. At this time, the inclusion of AlloP potentiated the benefits of Prempro®, but only among Tat(-) controls and Tat(+) post-estropausal mice. Tat-induced cognitive deficits were not observed until mice transitioned to post-estropause. Prempro® alone did not improve cognition in Tat(+) mice, but did so when combined with AlloP. Similarly, co-administration with AlloP improved antiociception in post-estropause.
Conclusion:Tat and endocrine aging exhibited independent and interactive effects to accelerate age-related comorbidities in female mice. Hormonal replacement therapy, including combined Pempro® and AlloP, provided the greatest benefit to post-estropausal mice exposed to HIV Tat. Including a neurosteroid, such as AlloP, in HT may benefit for older women living with HIV.
|
| |
|
 |
 |
|
|