 |
 |
 |
| |
PRECLINICAL STUDIES TOWARD A PHASE I/IIA TRIAL USING ANTI-HIV DUOCAR-T CELL THERAPY
|
| |
| |
CROI 2022 Feb 11-16
Kim Anthony-Gonda1, Alex Ray2, Yuge Wang3, Hang Su Ariele Block2, Danica Lee2, Sarah Kleinsorge-Block4, Jane Reese4, Marcos de Lima4, Dimiter S. Dimitrov5, Rimas Orentas1, Steven G. Deeks6, Harris Goldstein2, Boro Dropulić1
1Caring Cross, Gaithersburg, MD, United States, 2Albert Einstein College of Medicine, Bronx, NY, United States, 3Lentigen, Gaithersburg, MD, United States, 4University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 5University of Pittsburgh, Pittsburgh, PA, United States, 6University of California San Francisco, San Francisco, CA, United States
program abstract
Background:
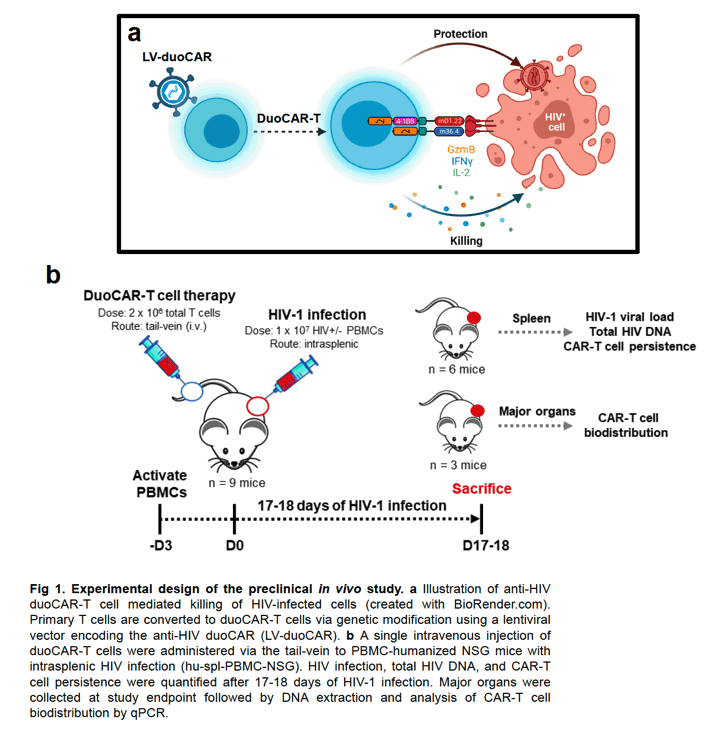
Anti-HIV chimeric antigen receptor (CAR) T cell therapies are candidates to functionally cure HIV infection in people with HIV (PWH). Paramount to translating such therapeutic candidates successfully into PWH will require anti-HIV CAR-T cells to traffic to lymphoid tissues in the body and eliminate reactivated HIV-infected cells. We hypothesized that clinical-grade anti-HIV duoCAR-T cells could traffic to the site of HIV infection in the spleen of humanized mice with HIV and potently suppress HIV infection.
Methods:
To test our hypothesis, we developed a GMP-complaint CAR-T cell manufacturing process using the CliniMACS Prodigy device to generate anti-HIV duoCAR-T cells at clinical scale. Clinical-grade anti-HIV duoCAR-T cells (2 x 10^6 total T cells) were intravenously injected into the tail-veins of PBMC-humanized NSG mice with intrasplenic HIV infection (hu-spl-PBMC-NSG). After 17-18 days of HIV infection, humanized mice were evaluated for signs of CAR-related toxicity and HIV infection quantified in the spleens of infected mice treated with and without duoCAR-T cell therapy.
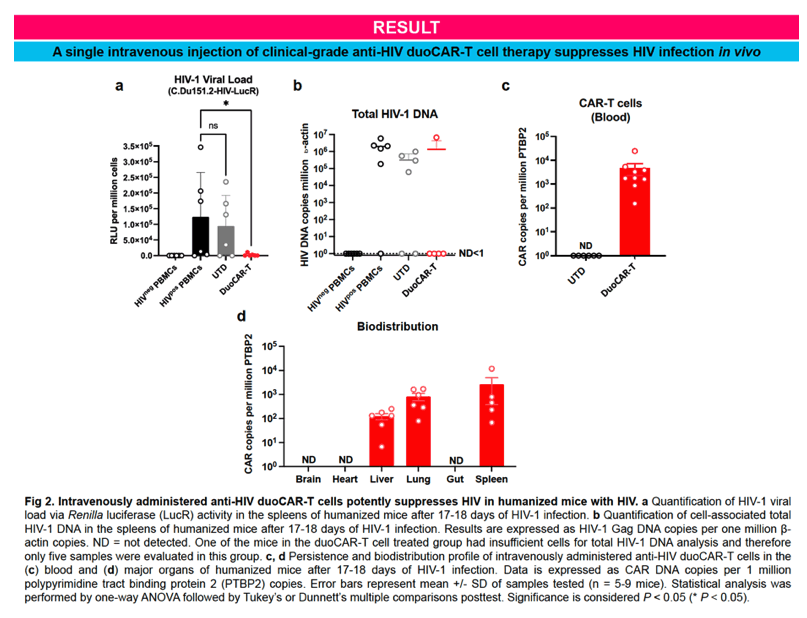
Results:
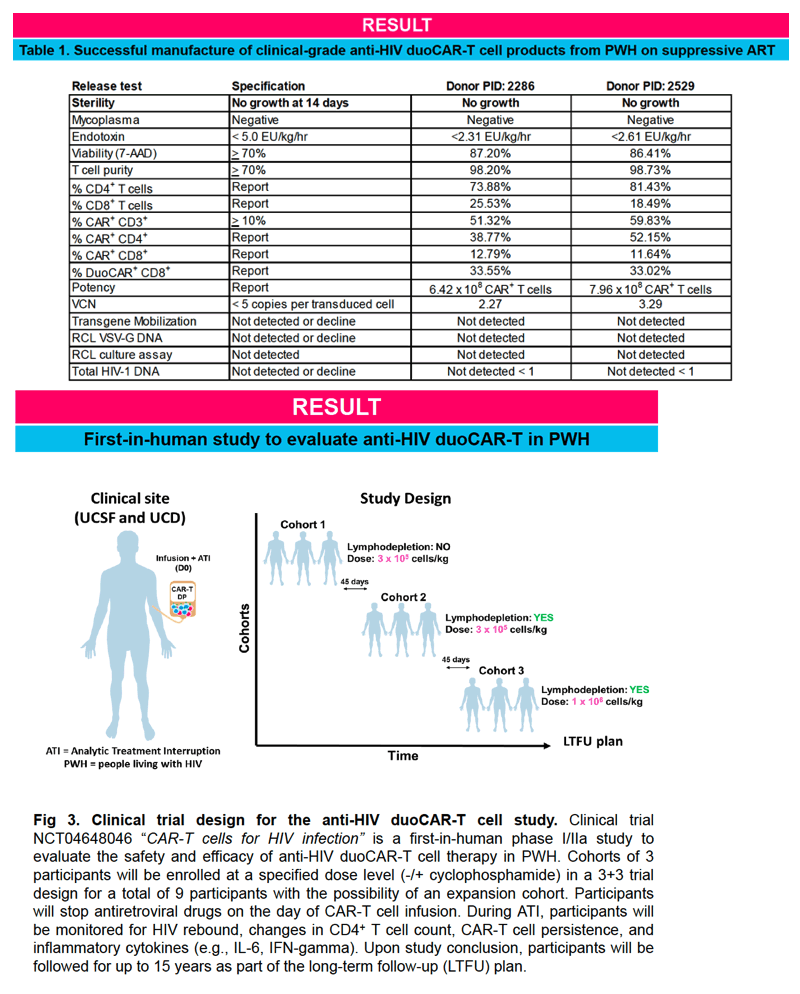
IND-enabling studies demonstrated that a single intravenous injection of clinical-grade anti-HIV duoCAR-T cells trafficked from the peripheral blood to the site of HIV infection in the spleen of mice with HIV and eliminated HIV-infected PBMCs. Anti-HIV duoCAR-T cells showed no apparent signs of CAR-related toxicity in humanized mice. Last, and in preparation for our clinical trial, we demonstrated our ability to successfully manufacture high quality anti-HIV duoCAR-T cell products from PWH in the absence of antiretroviral drugs using a GMP-complaint CAR-T cell manufacturing process.
Conclusion:
This work supports the initiation of our presently open phase I/IIa clinical trial (NCT04648046) to evaluate the safety and efficacy of anti-HIV duoCAR-T cell therapy in PWH.

|
| |
|
 |
 |
|
|