 |
 |
 |
| |
COUNTERFACTUAL ESTIMATION OF CAB-LA EFFICACY AGAINST PLACEBO USING EXTERNAL TRIAL DATA
|
| |
| |
CROI 2022 Feb 11-16
Deborah Donnell
Fred Hutchinson Cancer Research Center Seattle, WA, USA
program abstract
Background:
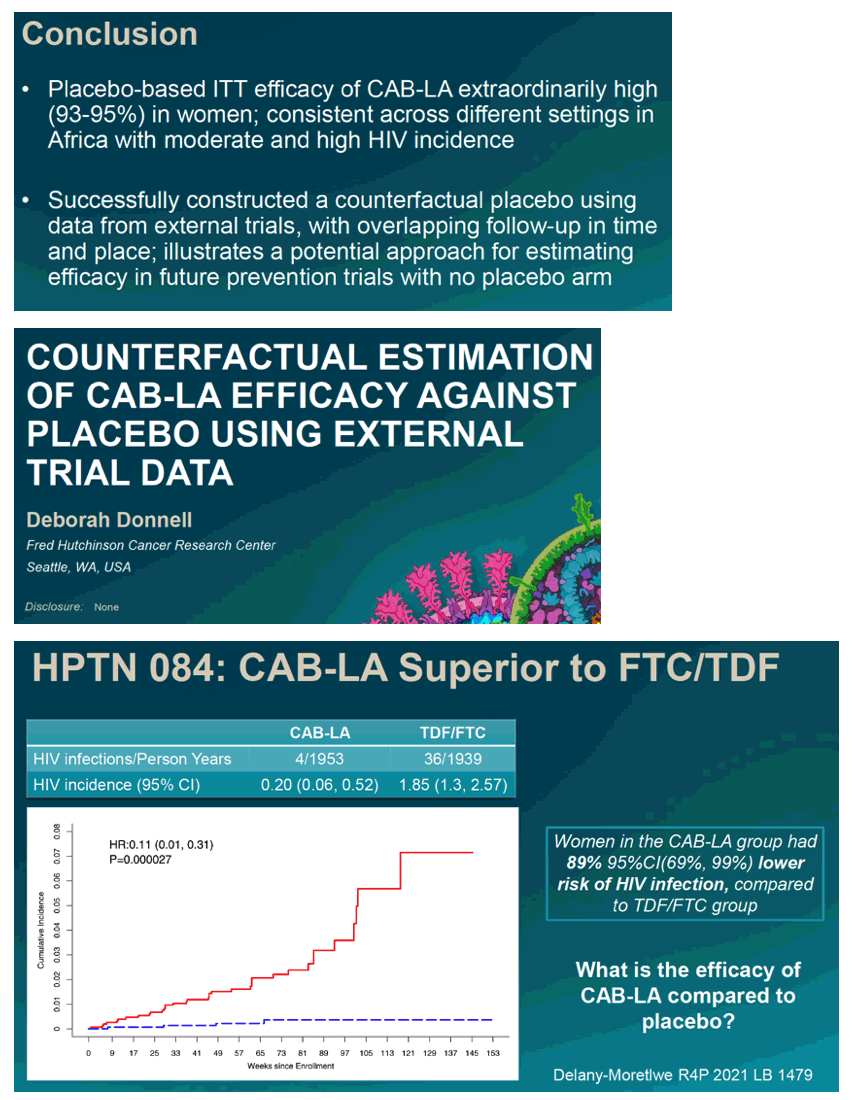
Oral PrEP is effective for HIV prevention in women, but daily adherence has proven challenging. HPTN 084 was an active-control randomized trial comparing oral PrEP (TDF/FTC) to the long-acting antiretroviral cabotegravir (CAB-LA) in women in sub-Saharan Africa. CAB-LA reduced HIV infection rates to very low levels, showing an 89% reduction relative to TDF/FTC, in follow-up between Nov2017-Nov2020. However, efficacy compared to no use of PrEP is unknown. The use of counterfactual placebo measures of incidence has been proposed to assess clinically useful estimates of PrEP efficacy.
Methods:
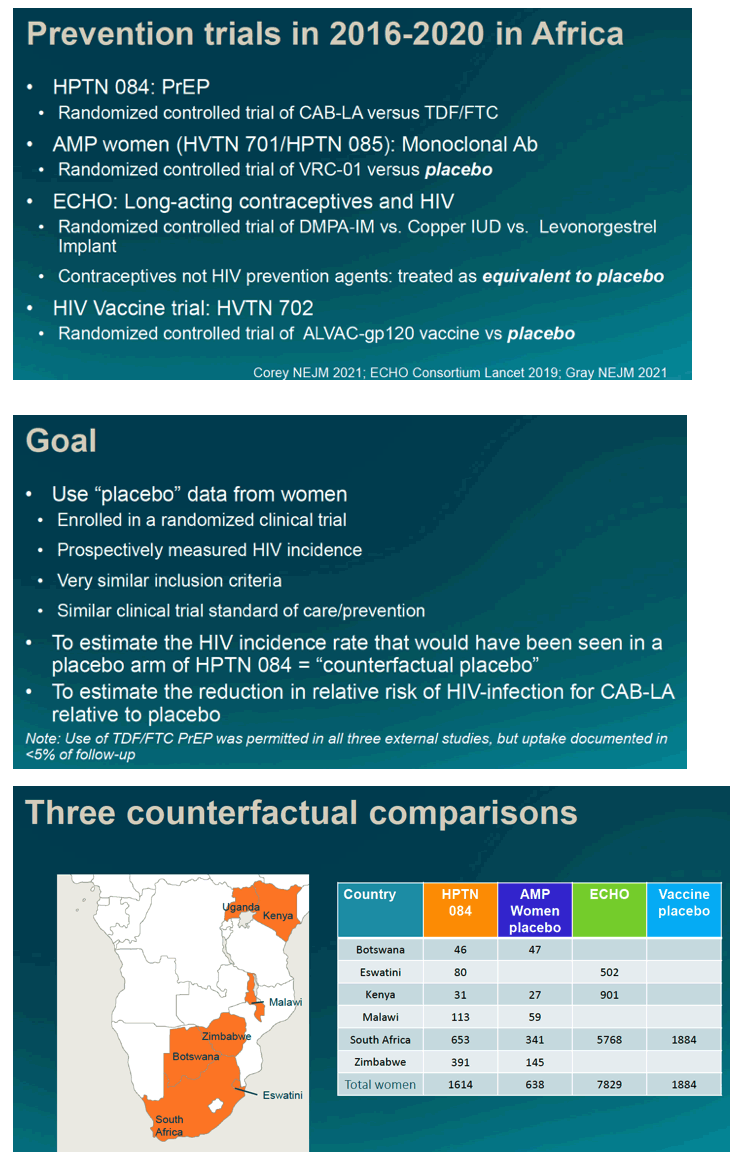
We used placebo arm data from women enrolled in three contemporaneous randomized HIV prevention trials to construct estimates of counterfactual (CF) placebo rates of HIV infection and efficacy for CAB-LA against a CF placebo. We construct estimates for each external trial, restricted to countries participating in both trials. Analysis weights were used to match relative person-years by country to HPTN 084 follow-up. The efficacy estimate compares incidence in the CAB-LA arm in HPTN084 to estimated counterfactual incidence, with confidence limits constructed assuming study independence and appropriately incorporating the use of weights. Data for women come from three trials conducted in southern Africa: the ECHO trial, the placebo arms of the AMP trial (HVTN 703/HPTN 081) and an HIV vaccine trial in South Africa (HVTN 702). Three counterfactual estimates were constructed, two in multi-country settings (AMP and ECHO) and one in South Africa (HTVN702). Oral PrEP was part of the standard of prevention in all three studies, but person-time with use of oral PrEP was less than 5%.
Results:
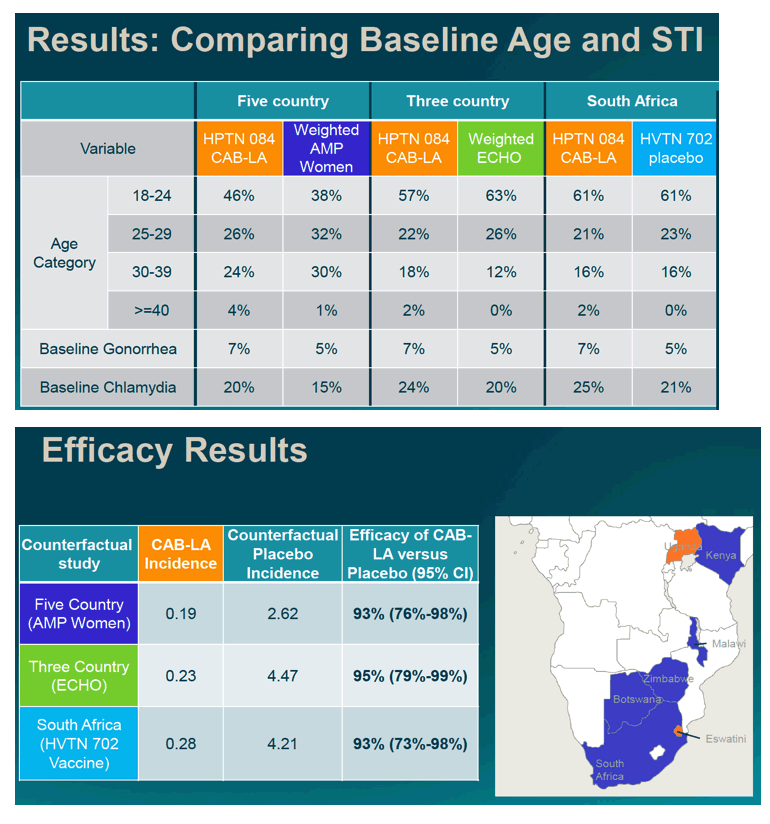
The placebo counterfactual studies included 637 women from AMP, 7829 from ECHO and 1884 from HVTN702; HPTN 084 had 1614 women in the CAB-LA arm. After analytic weighting, age, marital status and baseline rates of gonorrhea and chlamydia infection were similar between CAB-LA and placebo. Counterfactual placebo HIV incidence rates in women in the multi-country settings were 2.6/100PY (AMP), 4.5/100PY (ECHO) and 4.2/100PY (HVTN702); compared to 0.19/100PY, 0.23/100PY, and 0.28/100PY in the CAB-LA arm, respectively. Estimates of CAB-LA versus placebo efficacy consistently showed CF placebo-based efficacy of 93-95% (Table 1).
Conclusion:
Counterfactual placebo data from external trials across a range of settings provide strong support for high efficacy of CAB-LA for HIV prevention in women.

|
| |
|
 |
 |
|
|