 |
 |
 |
| |
PHASE I PK, SAFETY, AND ACCEPTABILITY STUDY OF A 90-DAY TENOFOVIR VAGINAL RING
|
| |
| |
CROI 2022 Feb 15
Albert Liu1, Holly Gundacker2, Barbra A. Richardson2, Beatrice Chen3, Craig Hoesley4, Ariane Van der Straten5, May Beamer3, Jennifer Robinson6, Cindy Jacobson3, Rachel Scheckter7, Katherine Bunge3, Andrea Thurman8, Gustavo Doncel8, Jeanna Piper9, Mark Marzinke6
1San Francisco Department of Public Health, San Francisco, CA, USA, 2Statistical Center for HIV/AIDS Research and Prevention, Seattle, WA, USA, 3University of Pittsburgh, Pittsburgh, PA, USA, 4University of Alabama at Birmingham, Birmingham, AL, USA, 5Astra Consulting, Kensington, CA, USA, 6The Johns Hopkins University, Baltimore, MD, USA, 7FHI 360, Durham, NC, USA, 8Contraception Research and Development, Arlington, VA, USA, 9National Institute of Allergy and Infectious Diseases, Baltimore, MD, USA
program abstract
Background:
Vaginal rings are promising for long-acting HIV prevention. Extended duration rings may reduce user burden, cost, and encourage adherence. We evaluated the safety, pharmacokinetics (PK), adherence, and acceptability of a 90-day tenofovir (TFV) vaginal ring.
Methods:
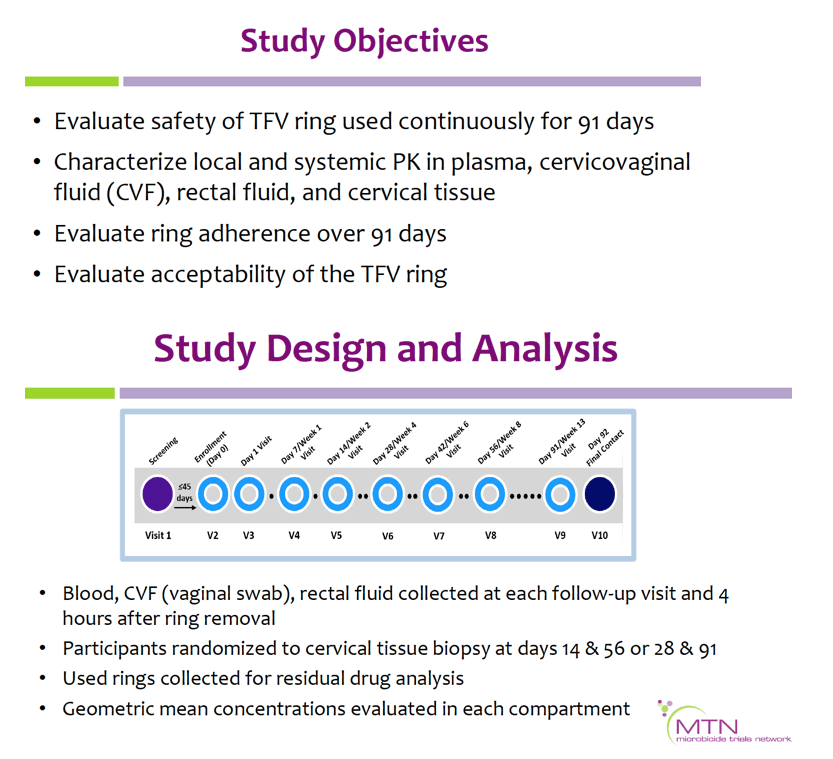
MTN-038 enrolled 49 HIV-negative participants into a Phase 1, multi-site, randomized (2:1) trial comparing a 90-day ring containing 1.4 grams (g) TFV used continuously to a placebo ring. TFV concentrations were quantified in plasma, cervicovaginal fluid (CVF), rectal fluid, and cervical tissue, and TFV-diphosphate (TFV-DP) in cervical tissue. Used rings were analyzed for residual TFV levels. Safety was assessed by adverse events (AEs); acceptability and adherence by self-report.
Results:
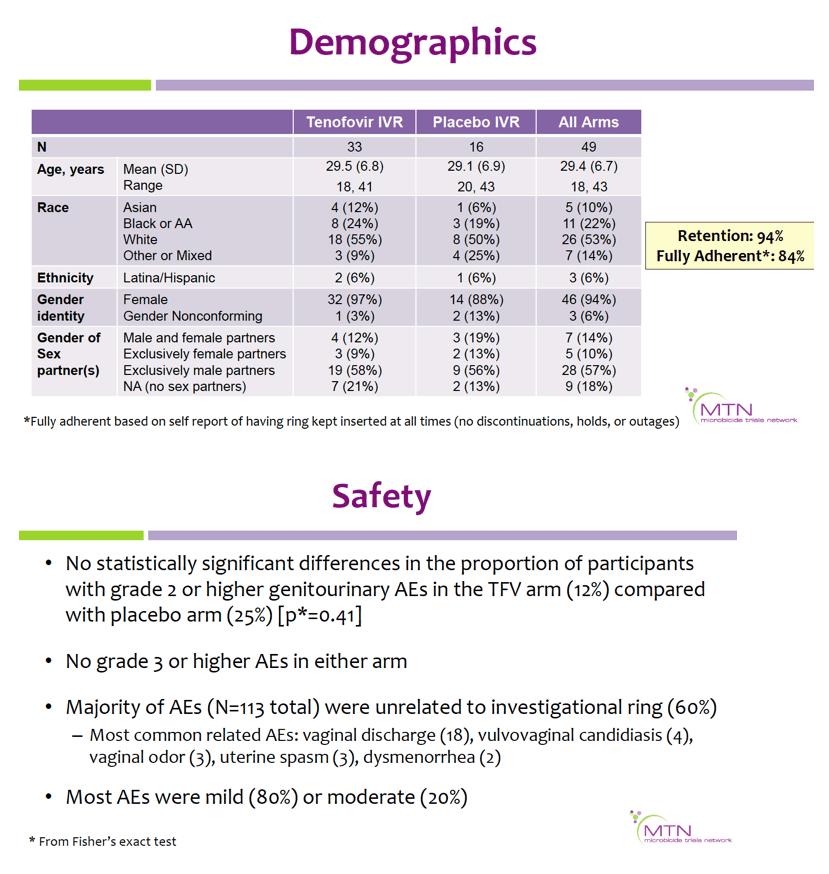
Mean age was 29.4 (range 18-43) years; 22% were Black, 53% white, 10% Asian, and 14% mixed/other race. Retention was 98%through day 91, and 84% reported being fully adherent. There were no differences in the proportion of participants with grade ≥2 genitourinary AEs in the TFV vs. placebo arms (p=0.41); no grade ≥3 AEs were reported.
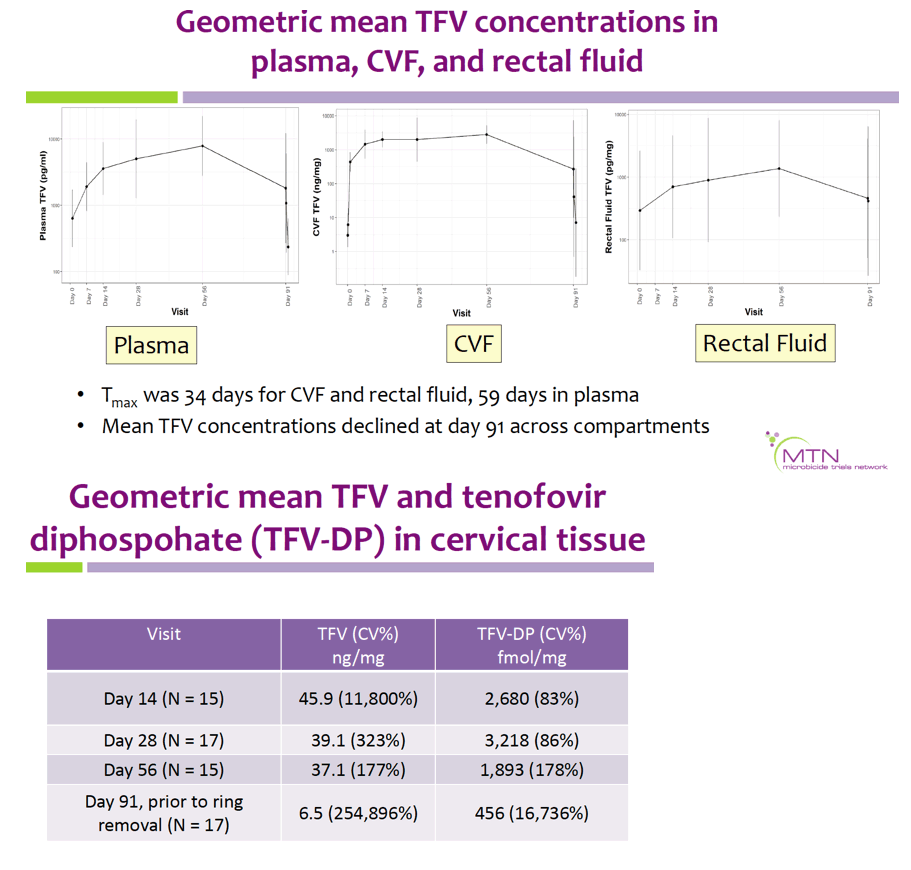
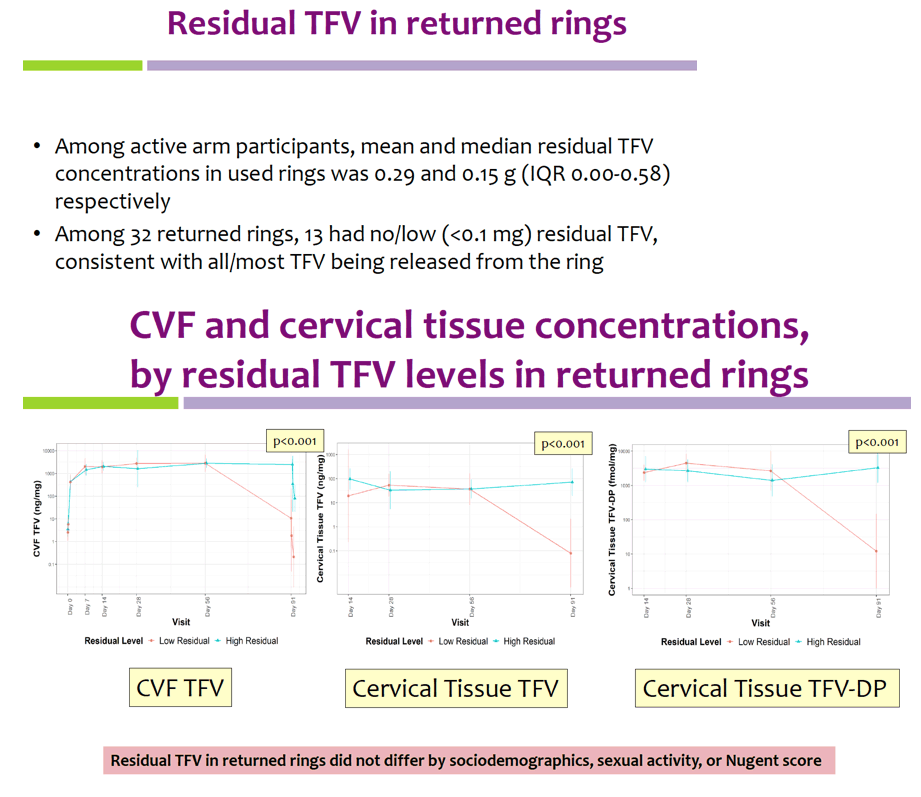
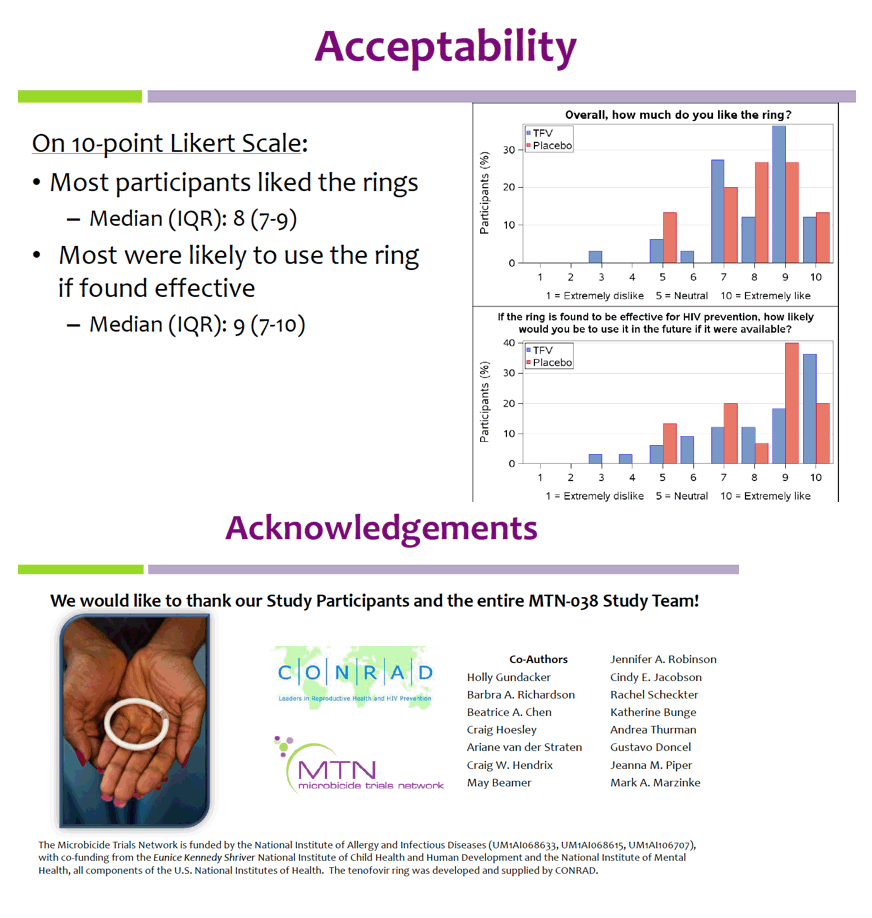
Geometric mean TFV concentrations and PK parameters are shown in the Table. Tmax was 34 days for CVF and rectal fluid, with mean TFV concentrations declining at day 91. Geometric mean TFV-DP tissue concentrations exceeded the 1,000 fmol/mg target through day 56, but fell to 456 fmol/mg by day 91. Mean and median residual TFV concentrations in used rings were 0.29 and 0.15 g respectively (IQR 0.00-0.58 g). Among 32 returned rings, 13 had no or low (<0.1 g) residual TFV. Participants with no/low residual TFV had lower geometric mean TFV concentrations in CVF (11 vs. 2471 ng/mg) and lower cervical tissue concentrations of TFV (0.1 vs. 72 ng/mg) and TFV-DP (12 vs. 3296 fmol/mg) at day 91 vs. those with higher residual TFV (all p<0.001), however concentrations at earlier time points were not significantly different between groups. Residual TFV in returned rings did not differ by sociodemographics, sexual activity, or baseline Nugent Score. A majority of participants reported liking the ring (median (IQR): 8 (7-9) on 10-point Likert scale) and reported high likelihood of using the ring in the future, if effective (median (IQR): 9 (7-10)).
Conclusion:
The 90-day TFV ring was well-tolerated, acceptable, and exceeded target cervical tissue concentrations through day 56, but declined thereafter. Additional studies should characterize the higher release from TFV rings in some participants and the optimal duration of use.
|
| |
|
 |
 |
|
|