 |
 |
 |
| |
Sotrovimab mAb Limits Progression to Severe COVID Regardless of Serostatus
|
| |
| |
2022 CROI, February 12-16 and 22-24, 2022
Mark Mascolini
In a placebo-controlled trial enrolling symptomatic COVID-19 outpatients, SARS-CoV-2-seronegative people getting the monoclonal antibody (mAb) sotrovimab instead of placebo had an 84% lower risk of hospital admission or death from any cause [1]. Among seropositive people, numerically fewer people randomized to sotrovimab than placebo got admitted to the hospital. But interpreting these results is hard because of the small numbers of people analyzed.
A phase 3, randomized, double-blind, placebo-controlled trial enrolling outpatients with mild or moderate COVID-19 and one or more risk factors for progression [2] found that a single 500-mg infusion of sotrovimab lowered risk of progression resulting in hospital admission or death [3]. Homing in on a highly conserved region of the SARS-CoV-2 spike protein, sotrovimab has activity against the Delta variant and the Omicron variant.
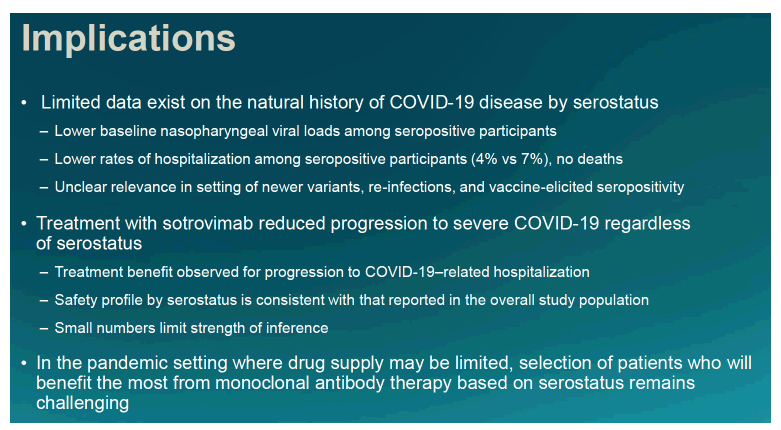
Understanding which people with mild or moderate COVID run a higher risk of progression could help clinicians focus treatments on patients most likely to be helped. Because some evidence suggested serostatus correlates with disease severity, sotrovimab researchers planned this analysis of the potential impact of serostatus on progression in the phase 3 trial.
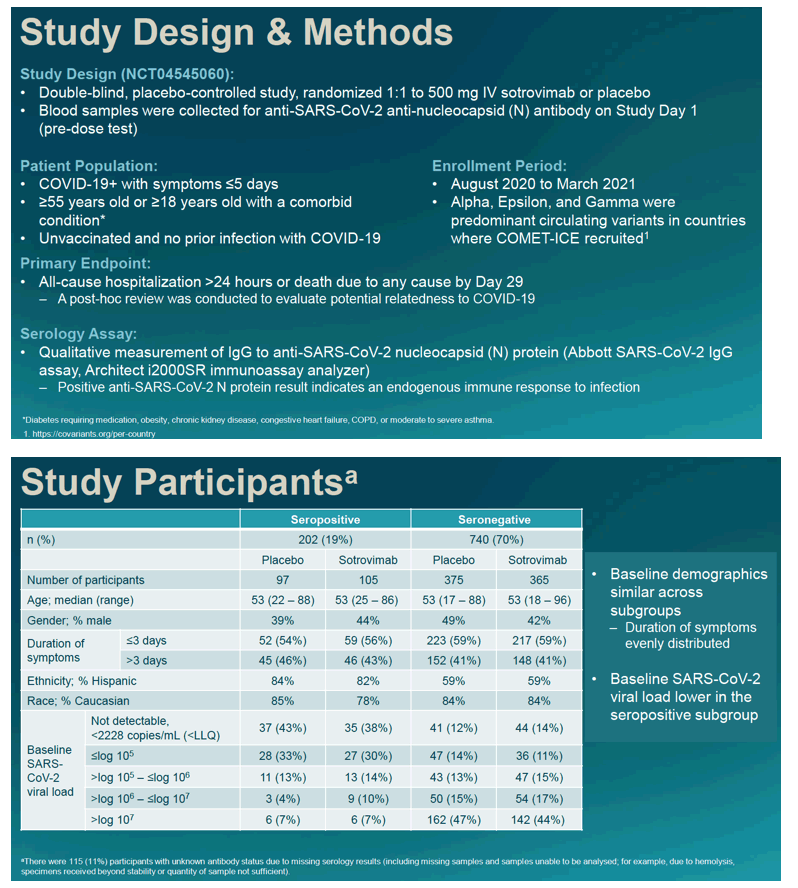
Study participants had to acquire COVID symptoms within the last 5 day. They had to be at least 55 years old, or at least 18 with a comorbid condition. They could not be vaccinated against COVID or have prior COVID. The primary endpoint was hospital admission longer than 24 hours for any cause or death from any cause by day 29. Researchers determined SARS-CoV-2 serology by measuring IgG to anti-SARS-CoV-2 nucleocapsid protein with the Abbott assay.
This analysis involved 202 seropositive people and 740 seronegatives. Median age stood at 53, and about 45% of participants were men. Slightly more than half of participants noticed COVID symptoms within the last 3 days.
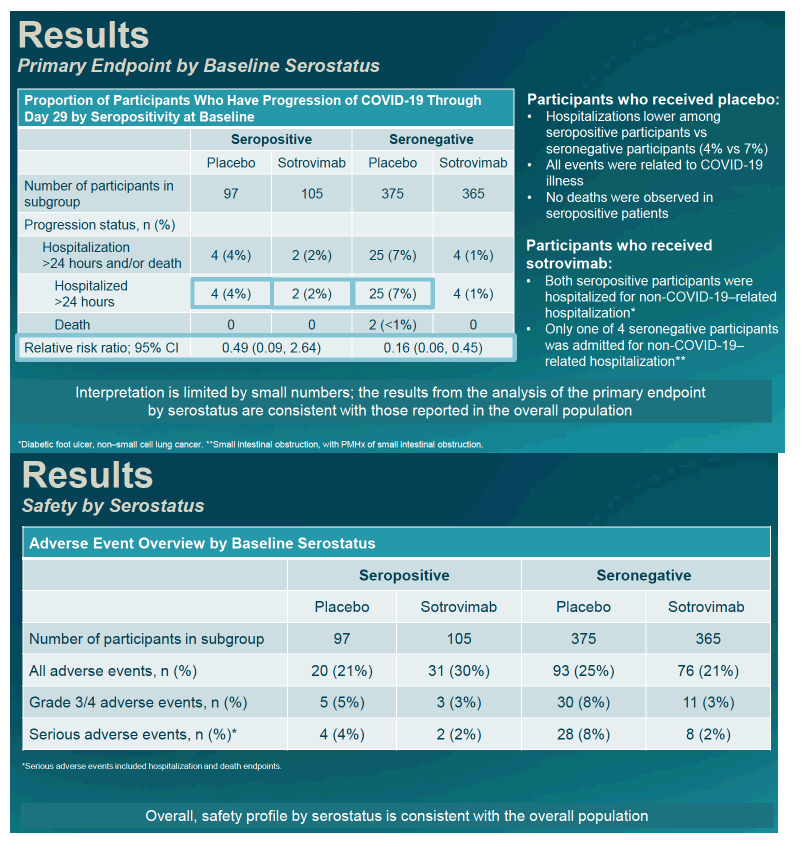
Among seronegative people, 4 (1%) who got sotrovimab got admitted to the hospital, compared with 25 (7%) who got placebo. No one getting sotrovimab died, compared with 2 people (under 1%) in the placebo group. Those numbers translated into an 84% lower risk of progression with sotrovimab in seronegative people (RR 0.16, 95% confidence interval 0.06 to 0.45). In the seropositive group, 2 (2%) randomized to sotrovimab and 4 (4%) randomized to placebo got admitted to the hospital. No one in either seropositive study arm died. The researchers noted that these results are tough to interpret because of the small number of participants.
Among seronegative participants, 3% randomized to sotrovimab and 8% randomized to placebo had grade 3 or 4 adverse events. Respective proportions with serious adverse events were 2% and 8%. Among seropositive people, 3% getting sotrovimab and 5% getting placebo had grade 3 or 4 adverse events, while 2% getting sotrovimab and 4% getting placebo had serious adverse events.
The researchers concluded that sotrovimab trimmed risk of progression to severe COVID regardless of SARS-CoV-2 serostatus. When COVID drug supplies are limited, they added, picking patients who will get the most benefit from a monoclonal antibody based on serostatus "remains challenging."
References
1. Gupta A, Sarkis E, Cathcart AL, et al. Effect of serostatus on efficacy of sotrovimab in preventing COVID-19 progression. 2022 CROI, February 12-16 and 22-24, 2022. Abstract 103.
2. ClinicalTrials.gov. VIR-7831 for the early treatment of COVID-19 in outpatients (COMET-ICE). ClinicalTrials.gov identifier NCT04545060. https://clinicaltrials.gov/ct2/show/NCT04545060
3. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941-1950. doi: 10.1056/NEJMoa2107934. https://www.nejm.org/doi/10.1056/NEJMoa2107934

|
| |
|
 |
 |
|
|