 |
 |
 |
| |
Leronlimab, mAb to CCR5, Trims Liver Fat in 14-Week Trial of NASH Patients
|
| |
| |
EASL International Liver Congress 2022, London, June 22-26, 2022
Mark Mascolini
Leronlimab (PRO 140), a monoclonal antibody (mAb) to human chemokine receptor 5 (CCR5), reduced liver fat in a 14-week placebo-controlled trial involving 72 adults with nonalcoholic steatohepatitis (NASH) [1]. At a subcutaneous dose of 350 mg weekly, leronlimab also lowered alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and markers of inflammation. Researchers who conducted the NASH01 Clinical Study believe leronlimab has multiple mechanisms of action in people with NASH.
Despite a high prevalence of NASH and risks posed by NASH-related fibrosis, no medications have been licensed to treat NASH. Recent work suggests that targeting chemokine-mediated inflammatory signaling pathways may lower liver fat and slow scar tissue formation in experimental fatty liver disease NASH [2].
In people with NASH, inflammatory cells from outside the liver get summoned to the liver injury site via interactions between cytokines or chemokines and their receptors. Ominously, people with NASH have high concentrations of CCR5 and its binder, CCL5-a finding suggesting CCR5 and its binders play a role in liver fibrosis in NASH patients. In NASH animal model studies, mAbs targeting chemokine signaling pathways had antiinflammatory and antifibrotic effects [3].
Researchers are developing leronlimab, a humanized mAb to CCR5, for HIV infection and other conditions. Studies in mice with NASH induced by a high-fat diet suggested leronlimab may also have a role in treating human NASH. To test that hypothesis, US and Canadian investigators conducted a two-part trial of adults with phenotypic evidence of NASH, a body mass index at or above 28 kg/m2, 8% or greater magnetic resonance imaging-N proton density fat fraction (MRI-PDFF), and iron-corrected T1 (cT1) at or above 800 msec at screening (inflammation and fibrosis markers) [4].
Trial part 1 randomized participants to 700 mg of leronlimab subcutaneously or placebo once weekly for up to 13 weeks. In part 2 all participants got 350 mg of leronlimab subcutaneously for up to 13 weeks. The research team set the primary study objective as change from baseline (pretreatment) hepatic fat fraction determined by MRI-PDFF at week 14.
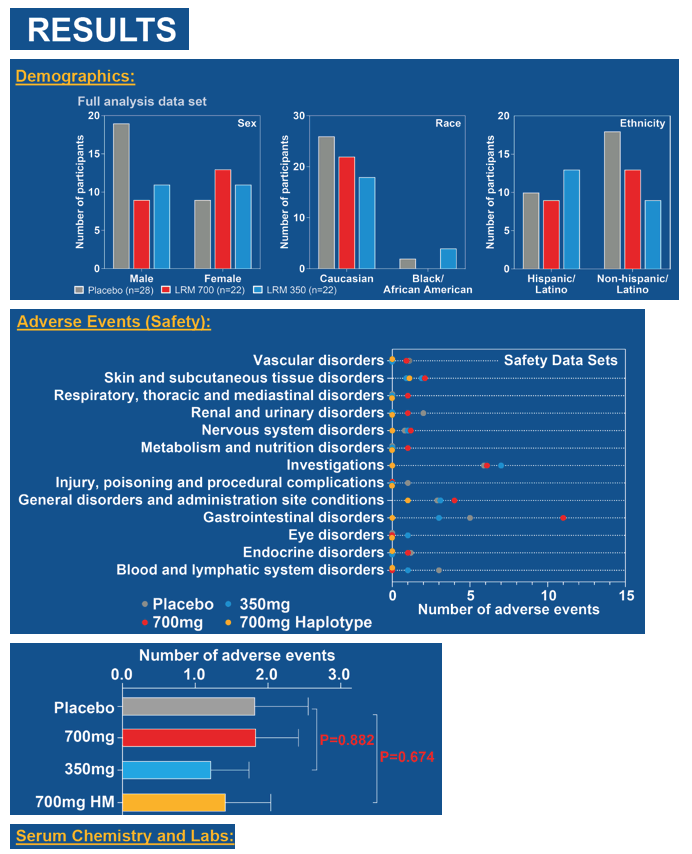
In the combined trial parts, 22 people received 700 mg of leronlimab weekly, 22 received 350 mg weekly, and 28 got placebo. Most study participants were Caucasian, with a balanced distribution of Hispanics and non-Hispanics. The trials included slightly more men than women. Numbers of adverse events did not differ much between the placebo group and either leronlimab dosing group.
Principal PDFF and cT1 results follow:
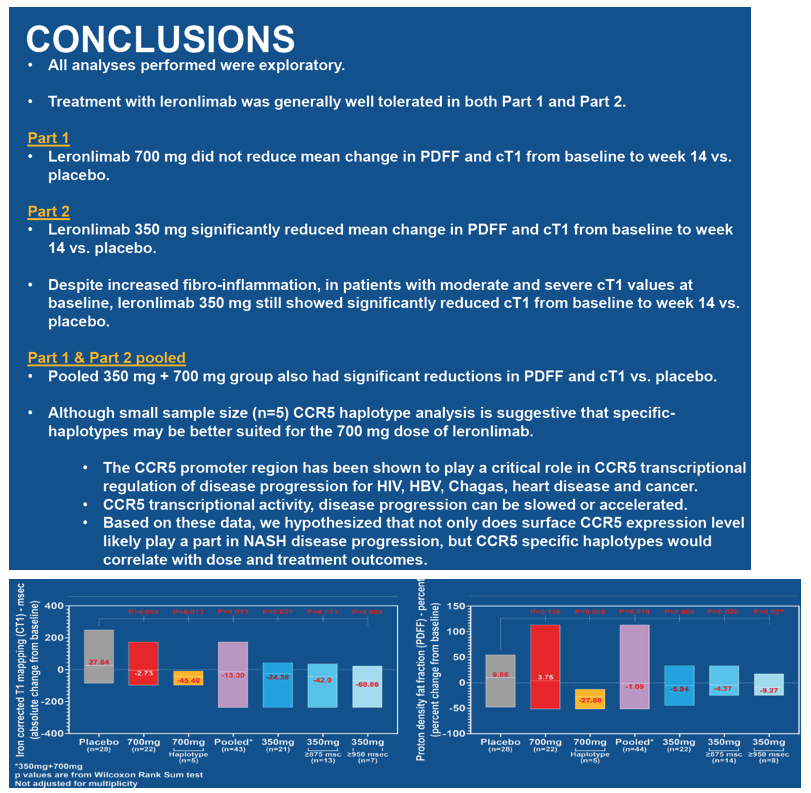
PDFF percent change from baseline
Placebo: 9.85
Leronlimab 700 mg: 3.75 (P = 0.136 vs placebo, not significant)
Leronlimab 350 mg: -5.94 (P = 0.008 vs placebo)
Leronlimab 700 and 350 mg pooled: -1.09 (P = 0.014 vs placebo)
cTI absolute change from baseline
Placebo: 27.64
Leronlimab 700 mg: -2.73 (P = 0.059 vs placebo, not significant)
Leronlimab 350 mg: -24.38 (P = 0.021 vs placebo)
Leronlimab 700 and 350 mg pooled: -13.39 (P = 0.013 vs placebo)
Compared with placebo, 700 mg of leronlimab weekly did not significantly lower average PDFF or cT1 change from baseline to week 14. But compared with placebo, 350 mg of leronlimab weekly did cut average PDFF and cT1 change from baseline to week 14. Pooled analysis of people in trial parts 1 and 2 also showed significant drops in PDFF and cT1 at week 14 compared with placebo. The 350-mg dose of leronlimab significantly lowered cT1 from baseline to week 14 even in participants with moderate or severe cT1 values at baseline.
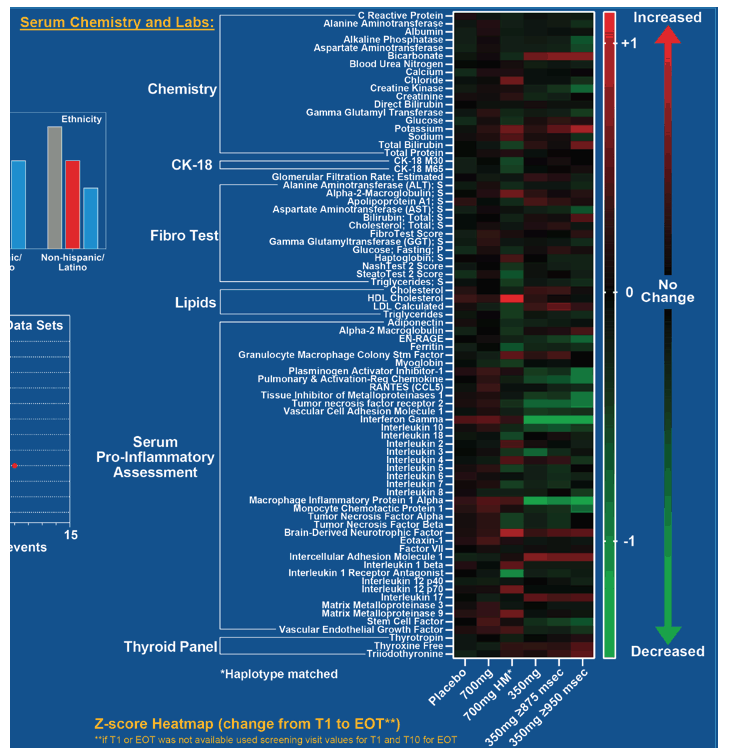
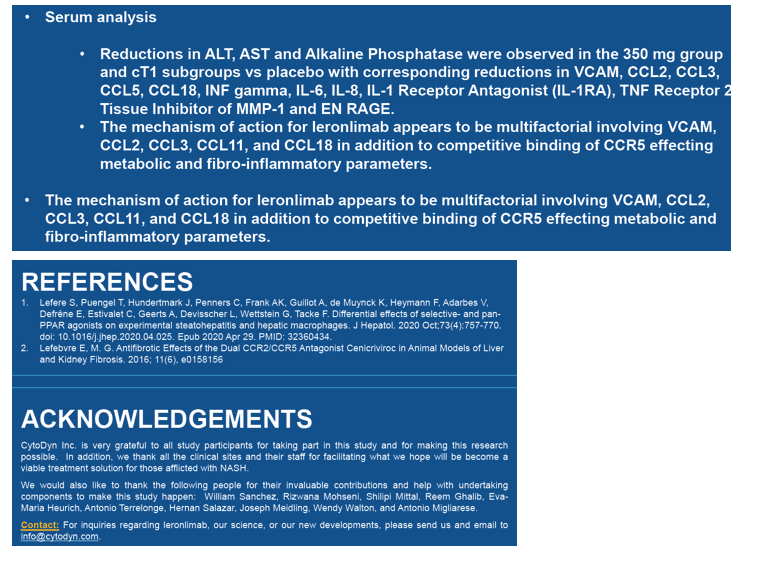
Compared with participants receiving placebo, those getting 350 mg of leronlimab weekly also had significant drops in ALT, AST, alkaline phosphatase and multiple markers of inflammation: VCAM, CCL2, CCL3, CCL5, CCL18, interferon gamma, IL-6, IL-8, IL-1 receptor antagonist. TNF receptor 2, and tissue inhibitor of MMP-1 and EN RAGE.
NASH01 investigators suggested leronlimab controls inflammation via multiple mechanisms involving VCAM, CCL2, CCL3, CCL11, and CCL18. Competitive binding of leronlimab to CCR5 may also contribute to antiinflammatory activity, they proposed.
References
1. Noureddin E, Lawitz A, Ritter T, et al. Efficacy and safety of leronlimab in patients with nonalcoholic steatohepatitis: topline results of NASH01 clinical trial. EASL International Liver Congress 2022, London, June 22-26, 2022. Abstract SAT101.
2. Lefere S, Puengel T, Hundertmark J, et al. Differential effects of selective- and panPPAR agonists on experimental steatohepatitis and hepatic macrophages. J Hepatol. 2020;73:757-770. doi: 10.1016/j.jhep.2020.04.025.
3. Lefebvre E, Moyle G, Reshef R, et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver. PLoS One. 2016;11(6):e0158156. doi: 10.1371/journal.pone.0158156. eCollection 2016.
4. ClinicalTrials.gov. Leronlimab (PRO 140) in patients with nonalcoholic steatohepatitis (NASH). ClinicalTrials.gov identifier NCT04521114. https://clinicaltrials.gov/ct2/show/NCT04521114


|
| |
|
 |
 |
|
|