| |
A phase 2 dose-finding study of lonafarnib and
ritonavir with or without interferon alpha for chronic delta hepatitis
|
| |
| |
----------------
Phase 3 Study:
Download the PDF here
Study of the Efficacy and Safety of Lonafarnib / Ritonavir With and Without Pegylated Interferon -Alfa-2a (D-LIVR)
https://clinicaltrials.gov/ct2/show/NCT03719313
The D-LIVR Study for the Treatment of Hepatitis D
Eiger BioPharmaceuticals Announces Complete Enrollment of D-LIVR, the Largest Phase 3 Study in Hepatitis Delta Virus (HDV), Investigating Lonafarnib, the Only Oral Agent in Development for HDV
-------------------------------
June 2022 - Cihan Yurdaydin1,2,3| Onur Keskin1| Esra Yurdcu2| Aysun Çalişkan1|Soner Önem1| Fatih Karakaya1| Çağdaş Kalkan1| Ersin Karatayli2,4|Senem Karatayli2,4|Ingrid Choong5| David Apelian5| Christopher Koh6|Theo Heller6| Ramazan Idilman1| A. Mithat Bozdayi2| Jeffrey S. Glenn7, 8
Abstract
Background and Aims
Proof-of-concept studies demonstrated lonafarnib (LNF), a first-in-class oral prenylation inhibitor, efficacy in patients infected with HDV. The lonafarnib with ritonavir for HDV-2 (LOWR-2) study's aim was to identify optimal combination regimens of LNF + ritonavir (RTV) ± pegylated interferon alpha (PEG-IFNα) with efficacy and tolerability for longer-term dosing. Here we report the safety and efficacy at end of treatment for up to 24 weeks.
Approach and Results
Fifty-five patients with chronic HDV were consecutively enrolled in an open-label, single-center, phase 2 dose-finding study. There were three main treatment groups: high-dose LNF (LNF ≥ 75 mg by mouth [po] twice daily [bid] + RTV) (n = 19, 12 weeks); all-oral low-dose LNF (LNF 25 or 50 mg po bid + RTV) (n = 24, 24 weeks), and combination low-dose LNF with PEG-IFNα (LNF 25 or 50 mg po bid + RTV + PEG-IFNα) (n = 12, 24 weeks).
The primary endpoint, ≥2 log10 decline or < lower limit of quantification of HDV-RNA from baseline at end of treatment, was reached in 46% (6 of 13) and 89% (8 of 9) of patients receiving the all-oral regimen of LNF 50 mg bid + RTV, and combination regimens of LNF (25 or 50 mg bid) + RTV + PEG-IFNα, respectively. In addition, multiple patients experienced well-tolerated transient posttreatment alanine aminotransferase increases, resulting in HDV-RNA negativity and alanine aminotransferase normalization. The proportions of grade 2 and 3 gastrointestinal adverse events in the high-dose versus low-dose groups were 49% (37 of 76) and only 22% (18 of 81), respectively.
Conclusions
LNF, boosted with low-dose RTV, is a promising all-oral therapy, and maximal efficacy is achieved with PEG-IFNα addition. The identified optimal regimens support a phase 3 study of LNF for the treatment of HDV.
INTRODUCTION
Chronic delta hepatitis (CDH) leads to the most severe form of human viral hepatitis. CDH is always found as a co-infection with HBV, requiring HBsAg to complete HDV virion assembly. However, HDV-HBV co-infection leads to more rapid disease progression than HBV mono-infection alone.[1] There is currently no Food and Drug Administration (FDA)-approved treatment for HDV, although the entry inhibitor Bulevirtide has recently received conditional approval by the European Medicines Agency. Pegylated interferon alpha (PEG-IFNα) has demonstrated limited efficacy in off-label use, in which 1 year of treatment with PEG-IFNα is effective in approximately only a quarter of the patients.[2] With prolonged treatment durations for up to 10 years, response rates may increase,[3] but this remains a difficult strategy for patients and physicians alike due to management of side effects. New therapies are urgently needed.
The farnesyl transferase inhibitor lonafarnib (LNF) targets prenylation of HDV large delta antigen, a host function that is essential for HDV virion morphogenesis.[4] By targeting a host function, LNF can subvert the ability of the virus to develop resistance, as the drug-targeted locus is not under genetic control of the virus. A proof-of-concept clinical study of LNF monotherapy demonstrated a dose-dependent correlation of increased LNF serum levels and HDV viral load (VL) decline at 4 weeks.[5] This was followed by the LOnafarnib With and without Ritonavir (RTV) in HDV (LOWR-1) study, which evaluated higher doses of LNF monotherapy as well as lower LNF doses boosted with RTV for up to 12 weeks' duration.[6] Although higher doses were associated with better short-term declines in VL, this was accompanied by increased gastrointestinal (GI) side effects. Higher LNF serum levels and antiviral responses were observed with lower LNF doses when combined with an inhibitor of its postabsorption metabolism, RTV. This enabled lower LNF doses in the GI tract to achieve higher efficacy with concomitant better GI tolerability. The present LOWR-2 study was designed to extend these findings to identify optimal combination regimens of LNF + RTV ± PEG-IFNα with efficacy and tolerability for longer-term dosing, to induce clinically meaningful reductions in HDV RNA and alanine aminotransferase (ALT) normalization, and to provide the support for the phase 3 study for patients infected with HDV.
DISCUSSION
In this study, three groups of LNF-based treatment regimens were assessed: high-dose Group 1 (LNF ≥ 75 mg bid + RTV), the low-dose all-oral Group 2 (LNF 25 or 50 mg bid + RTV), and the low dose combination Group 3 (LNF 25 or 50 mg bid + RTV + PEG-IFNα). The main findings of the LOWR-2 study can be summarized as follows: (1) All-oral therapy with LNF and RTV appears to be effective, and nearly half of patients on LNF 50 mg bid + RTV 100 mg bid reached the primary endpoint of the study; LNF 25 mg bid + RTV is well-tolerated but less effective; (2) combination therapy, consisting of the addition of PEG-IFNα 180 ug qw to the low-dose LNF treatment groups, displayed the most robust antiviral efficacy indicating synergy; (3) ALT normalization typically follows antiviral response; (4) most patients treated with LNF 25 or 50 mg bid + RTV ± PEG-IFNα were treated for a duration of 24 weeks; (5) for patients with low baseline viral loads, excellent responses are observed with all oral LNF 50 mg bid + RTV 100 mg bid; and (6) a subset of patients displayed a posttreatment ALT flare followed by HDV-RNA negativity and ALT normalization. Because a relatively small number of patients was subdivided into 10 treatment regimens, firm statistical conclusions were not, nor were they expected to be, reached. Nevertheless, the primary purpose of this study-to identify regimens with acceptable tolerability and efficacy to take into larger and longer studies-was accomplished.
Most patients treated with LNF in LOWR-2 demonstrated a decline in HDV-RNA levels at Week 24. Seven of 19 (36.8%) and 8 of 9 (88.9%) patients on all-oral low LNF + RTV and combination LNF + RTV + PEG-IFNα regimens, respectively, achieved ≥2 log10 decline (or < LLOQ) at end of treatment. The proportion of patients demonstrating HDV RNA with ≥2 log10 decline (or < LLOQ) at Week 24 increased with the LNF 50 mg bid dose (vs. 25 mg) and with addition of PEG-IFNα. One of 6 (16.7%) of patients treated with LNF 25 mg bid + RTV 100 mg bid achieved ≥2 log10 decline (or < LLOQ) at Week 24, which increased to 46.2% (6 of 13) with LNF 50 mg bid + RTV 100 mg bid. Addition of PEG-IFNα provided the most robust data to date, where 80% and 100% of patients (4 of 5 and 4 of 4) achieved ≥2 log10 decline (or < LLOQ) at Week 24 with LNF 25 mg bid + RTV 100 mg bid + PEG-IFNα 180 μg qw, and LNF 50 mg bid + RTV 100 mg bid + PEG-IFNα 180 μg qw, respectively. Overall, there was a better viral load response of the LNF 50 mg bid + RTV 100 mg bid group as compared with the LNF 25 mg bid + RTV 100 mg bid group. Addition of PEG-IFNα to either LNF 25 mg bid + RTV 100 mg bid or LNF 50 mg bid + RTV 100 mg bid therapy resulted in an improved virologic response as compared with the corresponding all-oral therapies.
Achieving the above ≥2 log10 decline (or < LLOQ) at EOT is important because such EOT responses have been associated with long-term survival benefit.[8] This latter study was based on treatment of CDH with conventional treatment for 1 year, whereas in the current study treatment duration was no more than 24 weeks, and a comparison with a study of longer treatment duration may not be appropriate. In addition, a ≥2 log10 decline (or < LLOQ) at EOT has been endorsed as the most appropriate surrogate for initial treatment efficacy for future registration studies in HDV by a panel of experts.[7]
Although combination treatment with PEG-IFNα demonstrated the most robust antiviral response, an all-oral regimen is an important option for patients who cannot tolerate PEG-IFNα or are treatment-refractory to PEG-IFNα In addition, all-oral LNF 50 mg bid + RTV has demonstrated good antiviral activity in the approximately 30%-40% patients who present with low baseline viral loads of ≤ 4 log10. Indeed, 6 of 7 (86%) patients with low baseline viral load achieved ≥2 log10 IU/ml decline (or < LLOQ) after 24 weeks of treatment. Moreover, at 24 weeks following treatment follow-up, 33% (2 of 6) of patients demonstrated a durable virologic response of ≥2 log10 decline (or < LLOQ) and 50% (3 of 6) of patients with elevated ALT at baseline had normal ALT. There are at least three mechanisms that appear to contribute to the antiviral efficacy of LNF. By directly inhibiting farnesylation of large delta antigen, LNF blocks the assembly and release of HDV virus particles.[4] As a consequence of not being secreted in the form of nascent HDV particles, large delta antigen can exert its transdominant effect on HDV-RNA genome replication.[9] Finally, the intracellular retention of large delta antigen that is associated with LNF treatment has been shown to induce innate immune responses in HDV-infected cultured cells.[10, 11]
Patients with low baseline viral loads had higher rates of HDV RNA ≥ 2 log10 decline (or < LLOQ) at end of treatment. Therefore, patients with low viral load at baseline may be considered for all-oral treatment with 50 mg LNF in combination with RTV.
Most patients with HDV with ALT > upper limit of normal who were treated with low-dose LNF + RTV ± PEG-IFNα normalized their ALT after only 24 weeks of treatment. Spontaneous normalization of ALT is rare in untreated patients with HDV. ALT is a highly useful peripheral surrogate biochemical marker for the extent of inflammation in the liver. Numerous data sets from studies in patients with chronic HBV demonstrate the strong association of treatment-induced normalization of ALT with improved necro-inflammation based on liver histology using validated central pathology assessments.[12, 13]
Three patients who did not achieve HDV responses at EOT experienced posttreatment flares and resulting HDV-RNA negativity with ALT normalization. Importantly, all of these episodes were well-tolerated without any signs of clinical decompensation. One of these patients had a baseline liver biopsy, allowing for assessment of the effect of this ALT normalization on liver histology, which demonstrated regression of fibrosis from baseline. This, along with similar posttreatment flare results,[6] represents data on regression of fibrosis with new compounds in patients with HDV, although we do realize that the number of patients is small. The fact that HDV is associated with rapid progression of fibrosis might explain why such regressions of fibrosis have been observed in relatively short periods of time. Importantly, this suggests that the high rates of ALT normalization observed with LNF 50 mg bid regimens (either as all-oral with RTV, or as combination therapy with PEG-IFNα) are also likely to result in improvements of liver histology. This would be in line with other studies that have demonstrated that removal of the underlying major trigger of inflammation with resulting prolonged ALT normalization is associated with histologic improvement and regression of fibrosis.[14]
Limitations of this study include its open-label, single-center, and nonrandomized nature. Furthermore, no quantitative HBsAg kinetics or pharmacokinetic data were available to evaluate in this study, including in patients with a posttreatment flare. In addition, a large number of dosing groups were explored to identify optimal dosing regimens, and these dosing regimens had relatively small numbers of patients, which led to lack of statistical power associated with the findings of this study. Future clinical studies with larger numbers of patients focusing on the key dosing regimens will enable more firm statistical evidence. In particular, the effect of extending the key low-dose regimens (i.e., low-dose LNF all oral, or in combination with PEG-IFNα) to 48 weeks of treatment is currently being rigorously evaluated in a randomized, placebo-controlled, phase 3 study of 400 patients, which should provide definitive and statistically sound data (NCT03719313).
Several approaches targeting different steps of the HDV life cycle are in clinical development for the treatment of HDV.[15] LNF is the only oral HDV treatment in phase 3 development and inhibits the critical step of large delta antigen prenylation, which is essential for HDV particle assembly.[4, 16]LNF has now been studied in over 120 patients, most of whom were in the LOWR-2 study described herein, as well as in the LOWR-3 and LOWR-4 studies.[17, 18] We previously reported that LNF was not associated with resistance development in patients treated with LNF for 12 weeks.[6] The absolute barrier to the development of resistance to a given host-targeting strategy can vary with each specific host target-virus pair.
The main aim of the current study using 10 subgroups was to create a regimen or regimens that combines efficacy with tolerability. Gastrointestinal side effects to LNF such as anorexia, nausea, diarrhea, and weight loss were clearly more common and intense with the all-oral high-dose regimens, leading to our decision not to consider high doses for future studies in CDH. Weight loss, an objective tool to assess gastrointestinal side effects, of Grade 2 toxicity according to CTCAE criteria within the duration of 12 weeks, during which high-dose all-oral LNF was given in Regimens 1 to 5, was observed in one-third of patients (5 of 15). Within the same duration, weight loss of Grade 2 toxicity was seen in 21% of patients (5 of 24) with the low-dose group. However, while discontinuations of the treatment regimen due to AEs was seen in 21% (4 of 19) of patients in the high-dose group, none of the patients (0 of 24 patients) in the low-dose all-oral regimens discontinued treatment. In the context of weight loss, Grade 2 toxicity is defined as weight loss of 10% to <20% of baseline weight. Treatment duration in regimens 5 to 10 was 24 weeks, which allowed us to assess trends in weight loss over time as a reflection of gastrointestinal AEs. There were 9 patients, including regimens 9 and 10, with weight loss of toxicity Grade 2 at Week 12. Of these 9 patients, 3 patients discontinued treatment after Week 12; in 1 patient, toxicity grade further increased to Grade 3. In another patient, toxicity grade did not change, whereas in 4 patients toxicity grade decreased to Grade 1. In 1 patient, weight determinations at Week 24 was unfortunately missing. Thus, Grade 2 toxicity within 12 weeks was-in the current study-either associated with continuation of the AE intensity, leading to premature discontinuation of treatment or further worsening of the AE in some patients, whereas in others, AEs either improved or did not change with treatment continuation, suggesting adaptation of these patients to the treatment regimen.
An interesting observation was seen in all patients receiving LNF with RTV. LNF led to an asymptomatic increase in platelet counts. The mechanism of this increase in platelets is unknown, but could be potentially very interesting, especially in a patient population in whom thrombocytopenia is common. Secondary or reactive thrombocytosis as a consequence of acute blood loss, iron deficiency anemia, hemolytic anemia, acute or chronic infection or inflammation, and drug reactions has been well described.[19] In the current study, the increase in platelet count was associated with a decrease in hemoglobin levels in some patients but not in others. Although the mechanism for a decrease in hemoglobin levels needs to be clarified, a potential inverse correlation between a decrease of hemoglobin levels and an increase in platelet counts was not observed. Another consideration is that the increase in platelet count is a direct effect of LNF.
The mechanism of a decrease in hemoglobin levels needs to be clarified, which was not done in the current study, partly because most patients were asymptomatic, and in patients with symptoms such as fatigue, there were other confounders such as diarrhea or concomitant peg-IFN use. Hemoglobin levels in some patients fell below 10g/dl, although at Week 24 such patients constituted a minority (4 of 36 patients [11%]). Hemolysis could not be excluded as a cause of anemia with certainty, as several hemolysis markers were not available to us, but there was no increase in indirect bilirubinemia.
The lack of a placebo group and the rather small number of patients necessitates avoiding overinterpretation. The currently ongoing phase 3 study will enable a more conclusive approach. It needs to be said, however, that myelosuppression has been reported as a common AE of farnesyl transferase inhibitors in patients with leukemia.[20] An effect in this context was not observed on white blood cells and obviously not on platelets, at least in the doses used in the current study.
Identifying therapeutic regimens capable of achieving on-treatment HDV-RNA declines of ≥2 log10, such as those described here, represents an important advancement for CDH. Thus, identifying candidate regimens that are sufficiently well-tolerated, and capable of achieving on-treatment HDV-RNA declines of ≥2 log10, are important findings of this study. The all-oral LNF 50 mg bid + RTV 100 mg bid appears to represent one such regimen. Similarly, the combination regimen of low-dose LNF + RTV + PEG-IFNα appears promising, demonstrating both apparent synergy and maximal antiviral efficacy. Because of its comparable antiviral efficacy but significantly improved tolerability,[21] replacing PEG-IFNλ for PEG-IFNα in the LNF + RTV + IFN combination regimen may therefore allow comparable efficacy with maximal tolerability-a hypothesis that has recently been successfully tested (clinicaltrials.gov NCT03600714).
Most importantly, the results of the current study have led to the identification of well-tolerated and efficacious regimens (e.g., all-oral LNF 50 mg bid + RTV 100 mg bid; LNF 50 mg bid + RTV 100 mg bid + PEG-IFNα 180 μg qw) that have now entered the pivotal phase 3 study (NCT03719313) designed to seek FDA registration for the treatment of HDV.
PATIENTS AND METHODS
Study design and participants
The study was a single-center, open-label, nonrandomized, uncontrolled, phase 2 pilot study. Safety and tolerability of EBP-994 (LNF) was a primary objective of the study. From a safety, pharmacokinetic, and pharmacodynamic perspective, no a priori assumptions were made as to the expected treatment effect and associated variability. All patients were enrolled in the Department of Gastroenterology of the University of Ankara Medical School. The study protocol and three amendments to the original protocol were approved by the University of Ankara Medical School Ethics Committee. Briefly, 18-year-old to 65-year-old patients with CDH infection, documented by a positive anti-HDV test of at least 6 months' duration and detectable HDV RNA by PCR within 3 months to study entry, were included. All patients were required to be HDV RNA-positive at baseline and have compensated liver disease. Platelet and white blood cell counts had to be ≥100,000 (x109/L) and 3000 (x109/L), respectively. Detailed inclusion and exclusion criteria are listed in Table S1 and the Supporting Materials. The study was approved by the University of Ankara Medical School and the Ministry of Health ethics committees. All patients gave written, informed consent.
Adverse events (AEs) of the treatment regimens were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.
RESULTS
Patient population
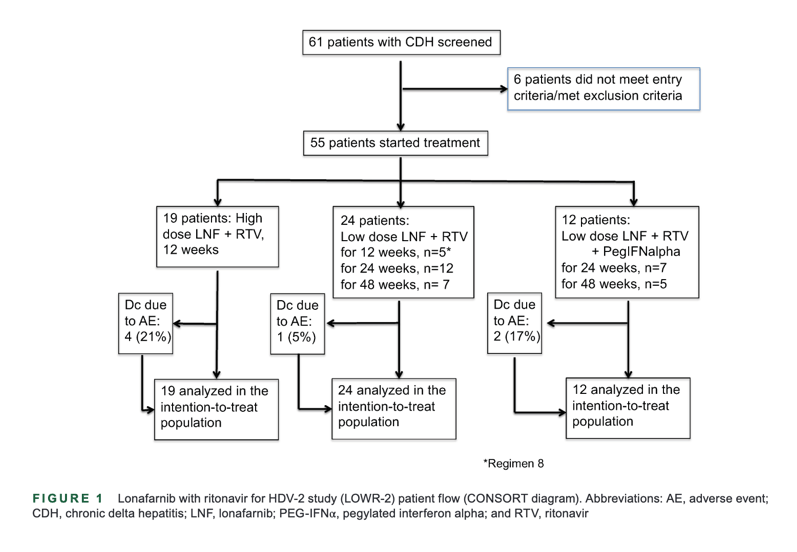
The study recruitment started December 2, 2014. The last patient was enrolled on October 25, 2016. Last per-protocol follow-up visit of a patient was on June 15, 2017. The study was closed after this date, as the planned dose finding had been achieved. A total of 55 patients were enrolled in the study (Figure 1). Of the 55 enrolled patients, 3 patients with platelet counts below the inclusion criteria threshold were enrolled as protocol deviation based on mutual agreement of the principal investigator (CY) and sponsor. There were four additional patients who were not protocol deviations, as their platelet counts were above 100,000 at screening but below the threshold at baseline (Regimen 5: 94,000; Regimen 6: 97,000 and 98,000; and Regimen 7: 72,000). Baseline patient characteristics are shown in Table 2. Briefly, most of the patients were HBeAg-negative, as is typically seen in HDV/HBV co-infection. Twenty-nine (53%) patients had historically received IFN treatment, and 26 (47%) patients were treatment-naïve. Median HDV-RNA levels were 4.56 log10 IU/ml (0.88-7.26). Median HBV-DNA levels were 1.71 log10 (range 0-7.76). There were 2 patients with HBV-DNA levels exceeding 4 log10 IU/ml; however, neither of these patients had viral loads suggesting HBV dominance. Sixteen (29%) patients had received prior nucleoside analog (NA) therapy. No patients received NA treatment during the study. Median ALT level was 67 U/l (24-651). Median platelet level was 154 (55-263). Of the 55 patients in the study, 14 (25%) had cirrhosis, 36 (65%) had chronic hepatitis without cirrhosis, and 5 (9%) could not be classified. Of the 14 patients with cirrhosis, all were Child-Pugh class A, and all but 1 had a Child Pugh score of 5 (Table 2).
Low-dose LNF (LNF ≤ 50 mg bid + ritonavir) has comparable efficacy with less side effects than high dose (LNF ≥ 75 mg bid + RTV)
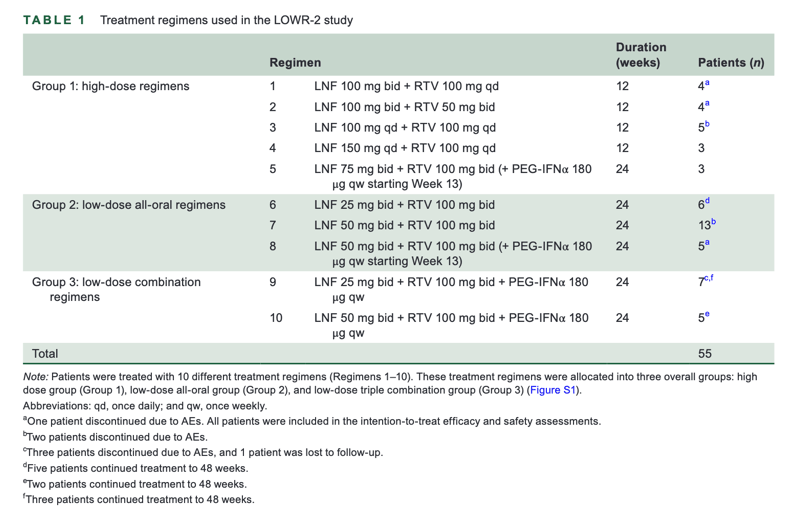
Initially, higher doses of LNF were explored in Regimens 1-5 (LNF 100 mg bid + RTV 100 mg qd, LNF 100 mg qd + RTV 100 mg qd, LNF 100 mg bid + RTV 50 mg bid, LNF 150 mg qd + RTV 100 mg bid, and LNF 75 mg bid + RTV 100 mg bid). Because a lack of resistance was confirmed through detailed sequence analysis of patients treated with LNF monotherapy in previous studies,[5, 6] lower doses of LNF were explored in Regimens 6-8 (LNF 50 mg bid + RTV 100 mg bid and LNF 25 mg bid + RTV 100 mg bid).
Efficacy results for patients completing 12 weeks per protocol treatments on these regimens are presented in Figure S2, Table S2, and Table 3. Corresponding AEs for patients receiving at least one dose (intention to treat) on these regimens are presented in Table 4, Table S3, and Table S4.
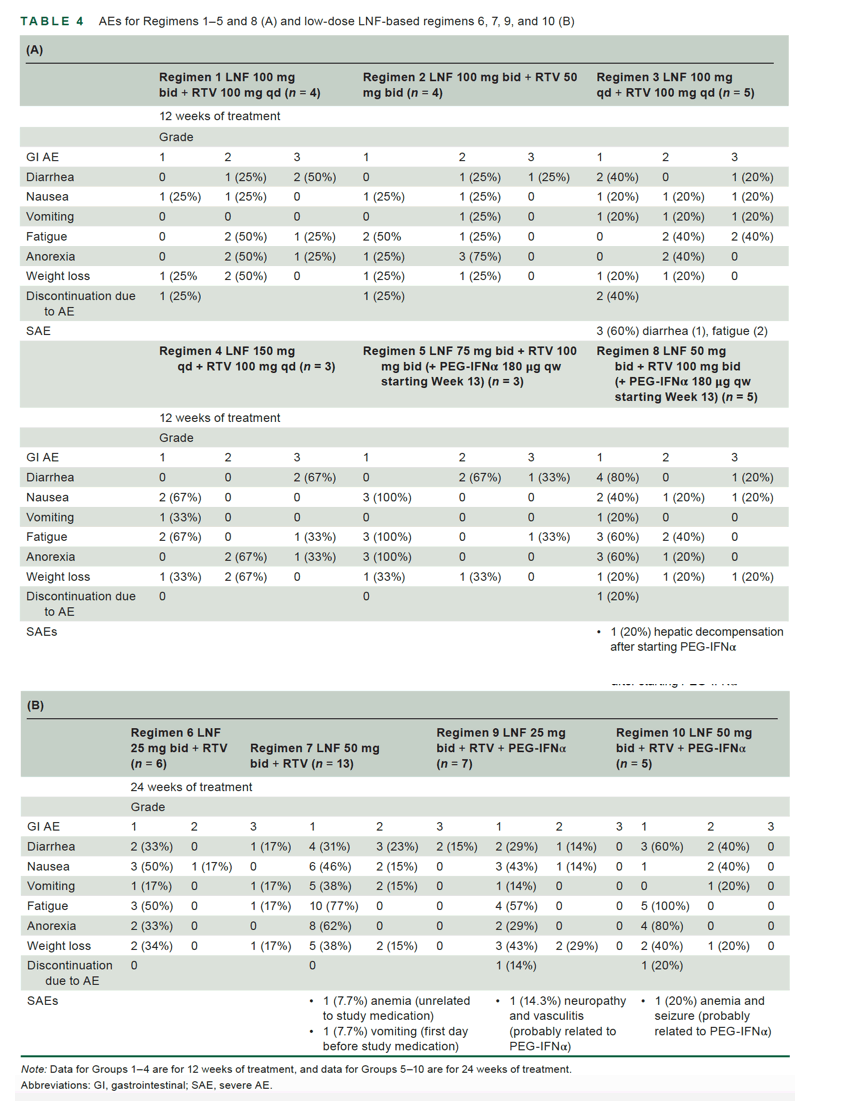
Week 12 observations from all oral LOWR-2 treatments indicated that high-dose LNF Group 1 patients (LNF ≥ 75 mg bid + RTV; Regimens 1-5; n = 19) had a mean viral load decline at Week 12 of -1.21 log10 IU/ml (Table S5A) with four discontinuations (21.1%), 11 dose reductions (57.9%), and GI AEs, 49% of which were Grade 2 or 3 in severity (Table S5B).
Low-dose LNF Group 2 (LNF ≤ 50 mg bid + RTV; Regimens 6-8; n = 24) had a mean viral load decline at Week 12 of -1.54 log10 IU/ml (Table S5) with discontinuation due to AEs in 1 patient and no dose reductions. In addition, GI AEs were predominantly Grade 1 (Table S5B). Baseline characteristics were comparable between these two groups (Table S6). No significant on-treatment elevations of liver enzymes, blood urea nitrogen, or creatinine were observed. With respect to severe AEs (SAEs), 3 occurred in the high-dose groups, probably related to LNF, and 5 in the low-dose groups-2 unrelated to study drug, 1 possibly related to PEG-IFNα, and 2 probably related to PEG-IFNα, with 1 patient experiencing hepatic decompensation after starting PEG-IFNα. No treatment-related or unrelated death occurred during the conduct of the study. Moreover, per-protocol analysis revealed that the number of patients with ≥2 log decline in HDV RNA after 12 weeks was 4 of 15 (27%) in the high-dose group and 14 of 24 (58%) in the low-dose group (Table S5A). (See Table S2 and Figure S2 for individual patient group data for patients reaching 12 weeks of treatment).
Given the comparable, if not better, efficacy, and fewer and less severe GI AEs with low-dose LNF versus high-dose LNF at Week 12 (Table S5), with comparable baseline characteristics between the two groups (Table S6), dosing durations of low-dose LNF regimens using LNF 25 and 50 mg bid were extended to 24 weeks, and the addition of PEG-IFNα was explored (i.e., Regimens 9 and 10 were added). Further analysis focused on these cohorts (Regimens 6, 7, 9, and 10) (Table 1).
Better antiviral efficacy was observed for the all-oral LNF 50 mg bid + RTV than the all-oral LNF 25 mg bid + RTV
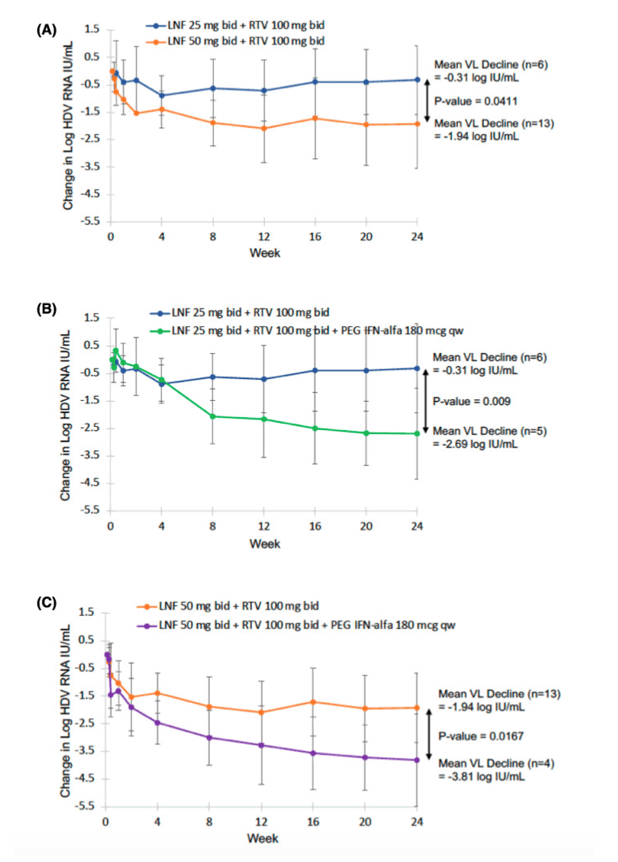
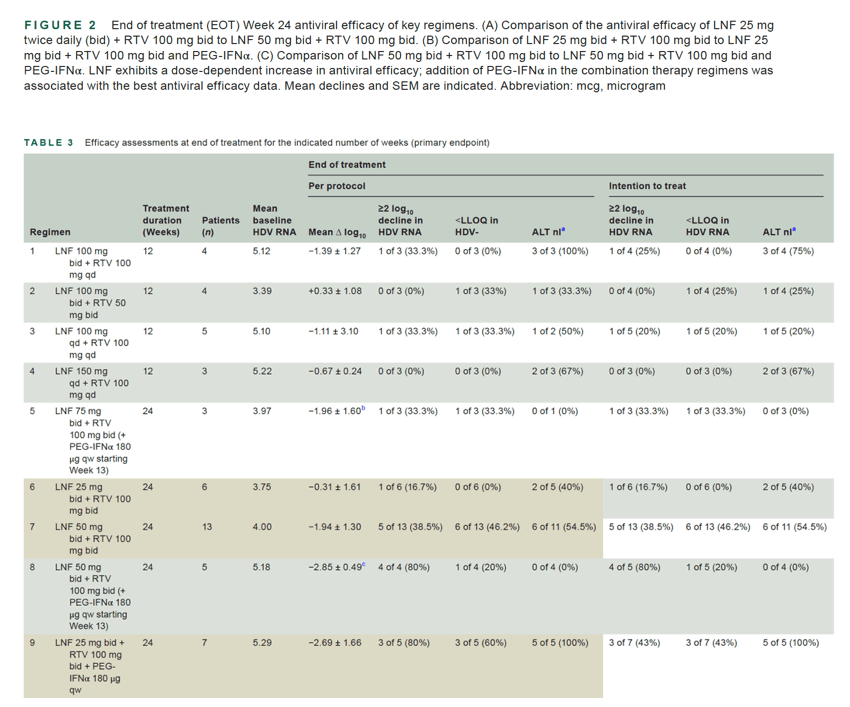
Assessment of the primary efficacy at 24 weeks of treatment revealed that LNF 50 mg bid + RTV 100 mg bid showed better antiviral efficacy compared with LNF 25 mg bid + RTV 100 mg bid. The latter regimen led to a mean HDV-RNA decline of -0.31 log10 IU/ml versus -1.9410 log IU/ml for the LNF 50 mg + RTV, an improvement of -1.63 log10 IU/ml (Figure 2A). Both regimens were generally well tolerated, with most side effects reported at the Grade 0-1 levels (Table 4). These AEs were generally more pronounced in the beginning of therapy and managed with pro-re-nata administration of proton pump inhibitors, ondansetron, and in some patients metaclopramide for nausea, and loperamide for diarrhea. The primary endpoint of ≥2 log10 decline (or < LLOQ) at 24 weeks EOT was reached in 1 of 6 (17%) patients in the lonafarnib 25 mg bid + RTV 100 mg bid group compared with 6 of 13 (46%) patients in the LNF 50 mg bid + RTV 100 mg bid group (Table 3). Of the patients with baseline elevated ALT, 2 of 5 (40%) patients receiving the LNF 25 mg bid regimen normalized their ALT at 24 weeks of therapy, whereas this was observed in 6 of 11 (55%) patients receiving the LNF 50 mg bid regimen (Table 3). Individual patient responses are indicated in Figures S3A,B, and S4A,B.
Combination LNF 25 or 50 mg + RTV + PEG-IFNα demonstrates the most robust antiviral efficacy
Combination therapy, consisting of the addition of PEG-IFNα to the LNF 25 and 50 mg bid RTV -boosted regimens, resulted in the most robust antiviral responses, with increased efficacy at 24 weeks compared with the respective all-oral regimens. In particular, combination therapy with LNF 25 mg bid + RTV 100 mg bid + PEG-IFNα 180 μg qw was associated with a -2.69 log10 IU/mL mean decline in HDV RNA, an additional decline of -2.38 log10 IU/mL over the LNF 25 mg + RTV 100 mg regimen (Figure 2B, Table 3). Addition of PEG-IFNα to LNF 50 mg bid + RTV 100 mg bid resulted in a -3.81 log10 IU/ml mean decline in HDV RNA, a further -1.87 log10 IU/ml decrease over the LNF 50 mg bid + RTV 100 mg bid regimen (Figure 2C, Table 3). Three of 5 (60%) patients on LNF 25 mg bid + RTV 100 mg bid + PEG-IFNα 180 μg qw achieved a ≥2 log10 IU/ml drop (or < LLOQ) after 24 weeks of therapy, and 5 of 5 (100%) patients with elevated baseline ALT normalized their ALT at 24 weeks of therapy (Table 3). Four of 4 (100%) patients on LNF 50 mg bid + RTV 100 mg bid + PEG-IFNα 180 μg qw achieved a ≥2 log10 IU/ml drop (or < LLOQ) after 24 weeks of therapy. All 3 patients with baseline elevated ALT normalized their ALT at EOT. Individual patient responses are indicated in Figures S3C,D and S4C,D. As expected, regimens with shorter durations of combination therapy (i.e., regimens 5 and 8; Table 1) had lower responses (Table 3). GI-related AEs, discontinuations due to AEs, and SAEs are summarized in Table 4 for all regimens. Effects on key hematologic and biochemical parameters are summarized in Table S3 and Figure 3. Effects on weight, dose modifications, and cumulative LNF dose are also included in Table S3. The most surprising finding from these analyses was a previously unrecognized effect of LNF on platelets (see Figure 3J). Indeed, lonafarnib exhibited an average 38% (p < 0.01) increase in platelet counts in all LNF-containing regimens (except those combining PEG-IFNα, which is known to be associated with cytopenias). This increase in platelets appears to be associated with a decrease in hemoglobin levels (Day 1: 14.4 ± 1.67 [x ± SD] g/dl vs. Week 12: 12.8 ± 1.86 [p < 0.0001]). An inverse correlation between the increase in platelet counts and decrease in hemoglobin levels, however, has not been observed (r: -0.289, p < 0.088 with Pearson correlation).
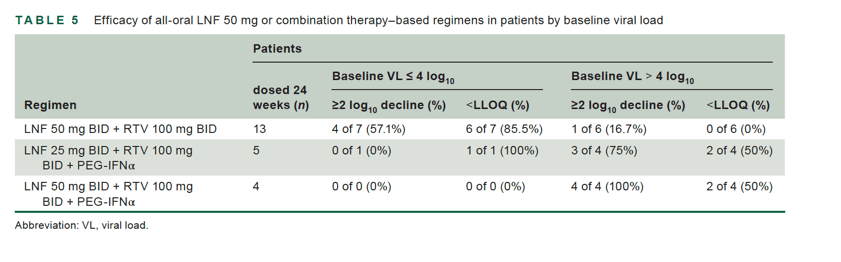
High response rates to all-oral LNF 50 mg bid + RTV in patients with low baseline viral loads
Patients with baseline viral loads ≤ 4 log10 demonstrated high response rates when treated with all-oral LNF 50 mg bid + RTV 100 mg bid, with 6 of 7 (86%) patients with ≥2 log10 IU/ml decline (or < LLOQ) after 24 weeks of treatment (Table 5). Only 1 patient with baseline low viral load received combination treatment with PEG-IFNα, making it difficult to draw any formal conclusion on the efficacy of triple combination treatment in low-viral patients, although this patient had a ≥2 log10 IU/ml decline (or < LLOQ) of HDV RNA after 24 weeks. Patients with baseline viral loads >4 log10, however, demonstrated high response rates when treated with LNF + RTV combined with PEG-IFNα, with 3 of 4 (75%) patients demonstrating ≥2 log10 IU/ml decline (or < LLOQ) with LNF 25 mg bid + RTV + PEG-IFNα, and 4 of 4 (100%) patients demonstrating ≥2 log10 IU/ml decline (or < LLOQ) with LNF 50 mg bid + RTV + PEG-IFNα after 24 weeks of treatment (Table 5).
|
|
| |
| |
|
|
|