| |
Hepatitis D virus in 2021: virology, immunology
and new treatment approaches for a difficult-to-treat disease
|
| |
| |
Stephan Urban,1,2 Christoph Neumann-Haefelin ,3 Pietro Lampertico 4,5
Abstract
Download the PDF here
Approximately 5% of individuals infected with hepatitis B virus (HBV) are coinfected with hepatitis D virus (HDV). Chronic HBV/HDV coinfection is associated with an unfavourable outcome, with many patients developing liver cirrhosis, liver failure and eventually hepatocellular carcinoma within 5-10 years. The identification of the HBV/HDV receptor and the development of novel in vitro and animal infection models allowed a more detailed study of the HDV life cycle in recent years, facilitating the development of specific antiviral drugs. The characterisation of HDV-specific CD4+ and CD8+T cell epitopes in untreated and treated patients also permitted a more precise understanding of HDV immunobiology and possibly paves the way for immunotherapeutic strategies to support upcoming specific therapies targeting viral or host factors. Pegylated interferon-α has been used for treating HDV patients for the last 30 years with only limited sustained responses. Here we describe novel treatment options with regard to their mode of action and their clinical effectiveness. Of those, the entry-inhibitor bulevirtide (formerly known as myrcludex B) received conditional marketing authorisation in the European Union (EU) in 2020 (Hepcludex). One additional drug, the prenylation inhibitor lonafarnib, is currently under investigation in phase III clinical trials. Other treatment strategies aim at targeting hepatitis B surface antigen, including the nucleic acid polymer REP2139Ca. These recent advances in HDV virology, immunology and treatment are important steps to make HDV a less difficult-to-treat virus and will be discussed.
Key messages
• At least 12 million individuals infected with hepatitis B virus (HBV) are coinfected with hepatitis D virus (HDV) and have a high risk to develop liver cirrhosis and hepatocellular carcinoma within a few years.
• Until 2020, there was no specific treatment option for the large majority of these patients; off-label use of pegylated interferon-α (pegIFNα) displays only approx. twenty per cent off-therapy virological response rates and is contraindicated in many patients.
• The identification of sodium taurocholate cotransporting polypeptide (NTCP) as cell entry receptor for both, HBV and HDV, allows the development of novel cell culture models and contributes to the development of novel treatment strategies.
• Beside de novo entry of virions via NTCP, cell division is an important mechanism of HDV spread; thus, combination therapies targeting these different mechanisms of viral spread are expected to show synergistic effects.
• Characterisation of HDV-specific immune responses will contribute to a better understanding of the mechanisms of HDV clearance versus persistence. It may also lead the way to novel treatment concepts, combining compounds that target host and/or viral targets and immunotherapeutic interventions.
• Three novel anti-HDV compounds target host factors: the entry inhibitor bulevirtide (BLV, Hepcludex), the prenylation inhibitor lonafarnib (LNF), and the nucleic acid polymer REP2139Ca.
• BLV was approved for conditional marketing in Europe in 2020. BLV in combination with (off-label) pegIFNα for 48 weeks or as monotherapy for a longer duration may allow sustained virological responses in a substantial proportion of patients.
• LNF and REP2139Ca also display encouraging response rates but additional data from phase III trials (ongoing for LNF) will be required prior to final assessment and possible approval by regulatory authorities.
Introduction
The human hepatitis D virus (HDV) is unique among animal viruses. Enveloped in the hepatitis B virus (HBV) surface proteins, HDV constitutes the smallest human virus with a diameter of 35-36 nm (figure 1A). HDV requires HBV as a helper for entry into hepatocyte, intrahepatic spread and dissemination between its hosts.1 2 Although recent in vitro findings indicate that HDV may propagate independent from HBV, using envelope glycoproteins from several virus genera such as vesiculovirus, flavivirus and hepacivirus including hepatitis C virus (HCV),3 clinical investigations confirm its strong association with HBV infection (hepatitis B surface antigen, HBsAg positivity).4-6 Some estimates suggest that up to 60 million individuals may be infected with HDV,7 8 however, another meta-analysis indicates that 12 million people are affected.9 HBV/HDV coinfection is associated with a more severe course of the diseases and an increased mortality compared with HBV monoinfection. Simultaneous infection with HBV and HDV of adults results in clearance of both viruses in the majority of individuals. In contrast, superinfection of an HBV-infected patient with HDV typically results in the development of persistent HBV/HDV coinfection which may lead to liver cirrhosis, liver failure and eventually hepatocellular carcinoma (HCC) within short time. Indeed, 50%-70% of patients with chronic HBV/HDV coinfection develop cirrhosis within 5-10 years after diagnosis, corresponding to a threefold increase compared with HBV-monoinfected patients.10 The risk for HCC development is increased compared with HBV monoinfection with an odds ratio (OR) of 1.28-2.77, depending on the selection of studies included in the meta-analysis.11 Due to this increased complication rate, HDV coinfected patients account for approx. 25% of HBsAg-positive liver transplant recipients in the European Liver Transplant Registry.10 Until recently, no approved antiviral treatment was available against HDV, thus, a more precise understanding of HDV virology and anti-HDV immune responses is essential to develop and establish novel therapeutic regimens.
Anti-hepatitis D treatment
Endpoints of therapy
The ideal endpoint for any anti-HDV therapy would be HBsAg loss with anti-HBs seroconversion. Elimination of replicating HDV RNA from the liver in HBsAg positive patients would be an alternative, however, it would require biopsies from patients and is not applicable in clinical practice. A more practical primary endpoint outcome is serum or plasma HDV RNA (as a surrogate marker of liver HDV-RNA levels) below the limit of detection by a sensitive and specific PCR assay during therapy, at the end of treatment (EOT) and off-therapy, at least 24 weeks after treatment discontinuation.72 However, given the high risk of late post-treatment virological relapses described after IFN-based therapies, a sustained off-therapy response should be confirmed over time, well beyond 24 weeks after treatment discontinuation. The proportion of patients with a≥2 log IU/ml decline of HDV RNA coupled with normal ALT have also recently been suggested as reasonable secondary endpoints for clinical trials.73 To comply with these stringent virological endpoints, it is of paramount importance to rely on commercially available, validated, WHO standardised, sensitive and specific HDV RNA assays that may allow to compare viral kinetics within as well as across studies.73
Current anti-HDV treatment
IFNα
Although not FDA or EMA approved, standard and pegIFNα treatments have been widely used as anti-HDV strategy in the last 20-30 years. PegIFNα is the only treatment regimen currently recommend by international guidelines.74-76 A 48-week course of weekly subcutaneous injections of pegIFNα suppresses HDV replication in approximately 20%-30% of the patients 24 weeks off therapy, yet with significant side effects. Continuous administration of IFN for more than 48 weeks may lead to a lower likelihood of disease progression, with HBsAg loss occurring in about 10% of these patients during long-term follow-up.77 Although the long term, off-treatment virological/biochemical response induced by IFN treatment has been associated with improved outcomes,72 73 IFN has limited use in clinical practice given the fact that this drug is contraindicated in elderly people or in those with autoimmune disease stigmata or with advanced or decompensated liver disease. Moreover, many patients have been already unsuccessfully exposed to standard or pegIFNα in the past. Combination of pegIFNα-2a with adefovir for 48 weeks78 or with tenofovir disoproxil fumarate (TDF) for 96 weeks did not significantly improve the off-treatment virological responses.79 Of note, in approximately 50% of week 24 off-treatment responders, a late virological relapse was observed, further challenging the long-term effectiveness of this treatment.80
The general failure of IFN treatment to induce a long-term (>24 weeks) sustained virological response on HDV may be due to the persistence of HDV in the liver even at very low HBsAg levels. This concept is supported by the observation that after liver transplantation, HDV can persist in the liver for many months even in the absence of liver HBV DNA/cccDNA and serum HBsAg and HDV RNA.81
New anti-HDV strategies: completed phase II studies
NAPs with pegIFNα
Phosphorothioate NAPs are oligonucleotides with a broad spectrum of inhibitory activity against several viruses, whose exact mechanism in HDV is still unknown (see previous section on virology).
In the non-randomised, open-label phase 2 REP301 study, 12 treatment-naïve non-cirrhotic patients with chronic HBV/HDV coinfection from Moldova received 500 mg of REP2139Ca intravenously once per week for 15 weeks, followed by 15 weeks of 250 mg REP2139Ca+pegIFNα, then followed by 33 weeks of pegIFNα monotherapy (overall 63 weeks of therapy).82 At 24-week off-treatment follow-up, 5 (42%) patients were HBsAg negative, 5 (42%) anti-HBs positive, 7 (58%) with HBV DNA <10 IU/mL and 7 (58%) with negative HDV RNA by Robogene assay. A marked increase in ALT levels was observed after the introduction of pegIFNα in five patients but all remained asymptomatic. All patients experienced at least one adverse event, mostly pegIFNα-related. The virological responses observed at week 24 off-therapy were confirmed when the off-treatment follow-up was extended up to 3 years.83
LNF with or without pegIFNα
LNF is an orally administered inhibitor of farnesyl-transferase that blocks the prenylation of L-HDAg, showing an intracellular accumulation of RNPs and a dose-dependent reduction of serum HDV RNA. To optimise the risk-to-benefit ratio of this regimen, LNF was combined with ritonavir (RTV) to enable achieving higher (fourfold to fivefold) systemic exposure while improving its gastrointestinal tolerability, and with pegIFNα to achieve a more profound inhibition of viral replication and HBsAg levels.
In the phase II LOWR HDV-2, 3 and 4 studies,84-86 the efficacy and safety of different doses of LNF+RTV in monotherapy or combined with pegIFNα administered for 12 or for 24 weeks were assessed. In summary, an all oral antiviral strategy based on LNF 50 mg two times per day+RTV 100 mg two times per day led to HDV RNA decline ≥2 log or below the lower limit of quantification (LLoQ) in 39% of patients (7 of 18) and ALT normalisation in 60% at week 24 (EOT) (tables 1 and 2). A combined therapy based on LNF+RTV+pegIFNα increased the EOT responses to 89% (8 of 9) and 78%, respectively (tables 1 and 2). To date, the off-treatment virological or biochemical response rates of these regimens are not available.
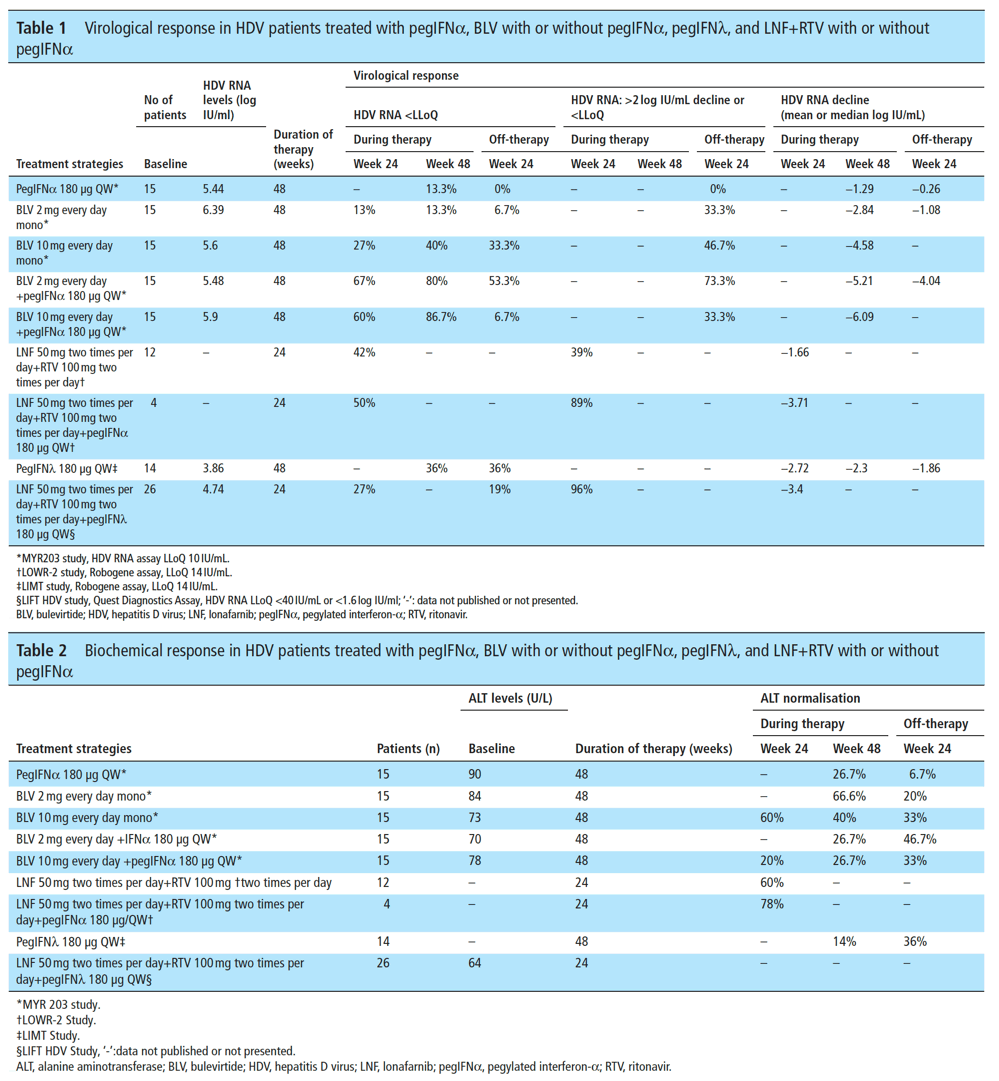
PegIFNλ with or without LNF+RTV
Lambda IFN binds a unique receptor vs type I IFN, highly expressed on hepatocytes, which may lead to a better safety profile of this compound compared with pegIFNα.
The phase II LIMT HDV study evaluated the safety, tolerability, and efficacy of subcutaneous pegIFNλ monotherapy administered at the dose of 120 vs 180 μg QW in addition to TDF/entecavir (ETV) in 30 patients with chronic HBV/HDV coinfection87 (tables 1 and 2). At week 72, by per-protocol (PP) analysis, the proportion of patients with undetectable HDV RNA, ALT normalisation and combined endpoints (ALT normalisation +≥2 log IU/mL HDV RNA decline vs BSL) was 16% vs 36%, 26% vs 36% and 11% vs 29%. Adverse events typically seen with pegIFNα were fewer, but 10% of patients experienced hyperbilirubinaemia and ALT/AST increases, that were reversible with dose reduction and without any signs of decompensation.
In the open-label phase II single arm LIFT HDV study, 26 HDV patients received LNF+RTV+pegIFNλ 180 μg QW for 24 weeks88 (tables 1 and 2). At week 24 (EOT), HDV-RNA decline was 3.2 (2.5-4.0) log IU/ml, 25/26 (96%) patients had a >2 log RNA decline and 11/26 (42%) patients had HDV RNA undetectable or < LLoQ. Dose reductions were required in 3/26 (11%) patients and treatment discontinuation in 4/26 (15%). At week 48, 24 weeks off-therapy, the virological response defined as HDV RNA <40 IU/mL by Quest Diagnostics assay was 19% (5 of 26 patients) by intention-to-treat (ITT) analysis and 23% (5 of 22) by PP analysis.89
BLV with or without pegIFNα
BLV, previously named Myrcludex-B, and approved in 2020 in Europe under the branded name of Hepcludex, is a subcutaneously delivered lipopeptide that mimics the NTCP RBD of the L-HBsAg, inhibiting the HBV/HDV entry into the hepatocytes (see previous section on virology).
Short-term therapy
In the multicentre phase IIb MYR202 study,90 120 TDF-treated patients with chronic HBV/HDV coinfection were randomised to different BLV doses (2, 5 or 10 mg/day) or TDF monotherapy for 24 weeks. A 2-log decline or undetectable HDV RNA at EOT (week 24) was reached by 46%, 47% and 77% of the patients treated with increasing doses of BLV but only in 3% of those on TDF monotherapy. ALT normalised in 43%, 50%, 40%, and 6%, while HBsAg levels were not affected. BLV was well tolerated, and elevation of glycine-conjugated and taurine-conjugated bile salts had no clinical consequences. An HDV-RNA relapse occurred in 60%, 80% and 83% of EOT HDV-RNA responders and was associated with a moderate increase in ALT levels.
In the phase II MYR203 study91 treatment with BLV, at different doses and with or without peg-IFN, was extended to 48 weeks (tables 1 and 2). 90 patients with chronic HBV/HDV coinfection were randomised into six treatment arms. The primary efficacy endpoint, HDV RNA below the LLoD (10 IU/mL) at week 72 (24 weeks off-therapy), was achieved by 0%, 53.3%, 26.7%, 6.7%, 6.7% and 33.3% of patients randomised to pegIFNα 180 QW, 2 mg BLV+pegIFNα, 5 mg BLV+pegIFNα, 5 mg BLV, 10 mg BLV+pegIFNa and 10 mg BLV, respectively. The corresponding ALT normalisation rates were 10%, 53.8%, 33.3%, 23.1%, 35.7% and 35.7%. HBsAg loss or > 1 log IU/ decline at week 72 was observed only in patients treated with BLV combined with pegIFNα: 40% for BLV 2 mg, 13.3% for 5 mg and 13.3% for 10 mg. BLV was generally well tolerated; an asymptomatic dose-related increase of total bile acids was observed. Most adverse events were observed in patients treated with pegIFNα, without any difference between patients treated as a monotherapy or in combination with BLV.
Long-term therapy
Two patients with compensated HDV-related cirrhosis, one with oesophageal varices, were treated with BLV monotherapy 10 mg for up to 3 years.71 Both cases normalised ALT levels before week 28 and achieved undetectable HDV RNA (<6 IU/mL by Robogene assay) before week 52. Biochemical and virological responses were maintained over 3 years of BLV administration without relapse or breakthrough, even after dose reduction of BLV from 10 to 5 and 2 mg/day.92 In the patient with more severe liver disease, virological response was associated with an excellent clinical response: oesophageal varices disappeared, histological/laboratory features of autoimmune hepatitis secondary to HDV infection resolved, AFP levels normalised and liver stiffness, platelets and albumin levels significantly improved. As far as safety is concerned, only asymptomatic, dose-related increase of total bile acids was observed. The optimal duration of long-term therapy with BLV monotherapy is at present unknown although application of an HCV-based kinetics model to HDV-infected patients suggests that after 3 years of continuous suppression of HDV replication by BLV more than 50% of the patients might achieve a long-term off-therapy virological response, despite persistence of HBsAg 93 and unpublished data).
New anti-HDV strategies: ongoing phase III studies
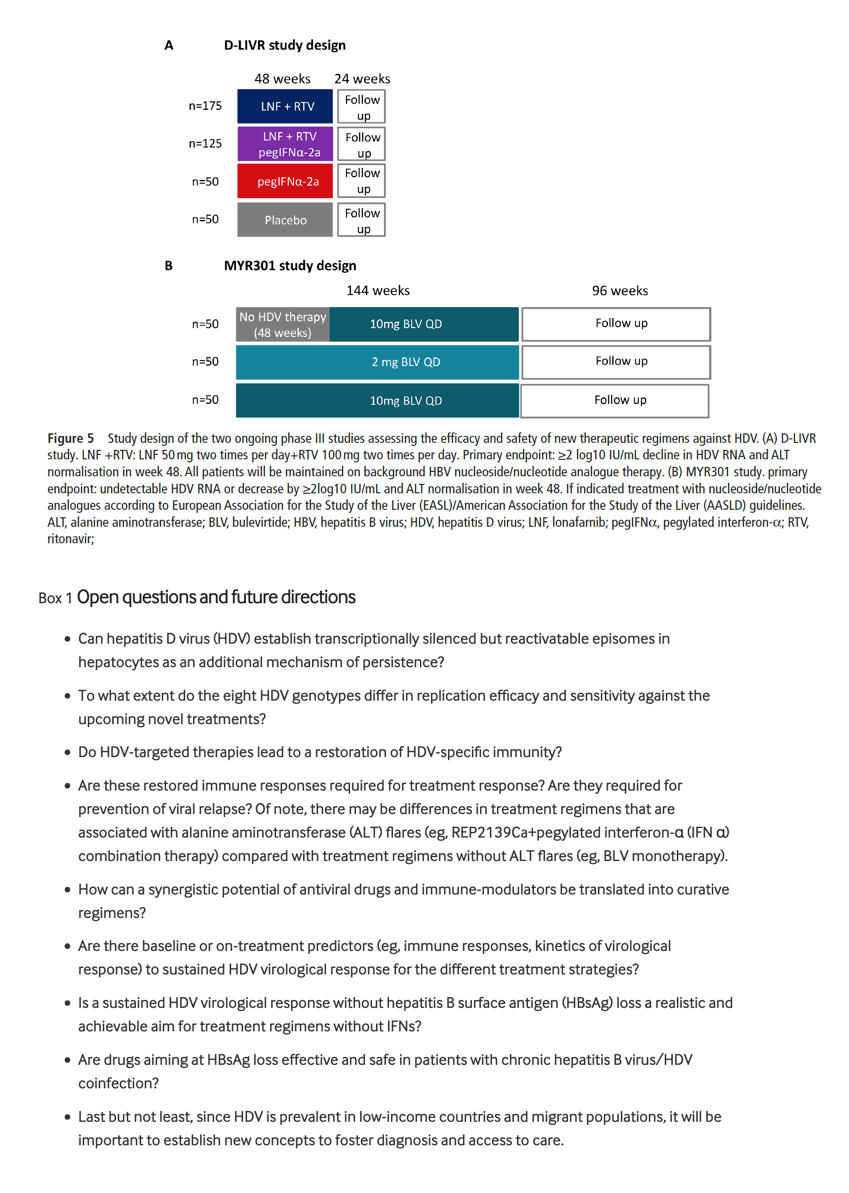
Two multicentre international phase III registration studies are ongoing (figure 5). In the D-LIVR study (EIG-LNF-011), 400 patients with chronic HBV/HDV coinfection will be randomised to four treatment arms (figure 5A) for 48 weeks. The primary endpoint is the proportion of patients achieving a≥2 log10 IU/ml reduction in serum HDV-RNA level and ALT normalisation at week 48 (EOT). In the MYR301 study, 150 patients with chronic HBV/HDV coinfection have been randomised to three different arms (figure 5B). The primary objective is to evaluate the safety and efficacy of different doses of BLV monotherapy administered up to 144 weeks. The total duration of the study is 240 weeks.
|
|
| |
| |
|
|
|