| |
Optimizing lonafarnib treatment for the
management of chronic delta hepatitis: The LOWR HDV-1 study
|
| |
| |
Download the PDF here
20 November 2017
Abstract
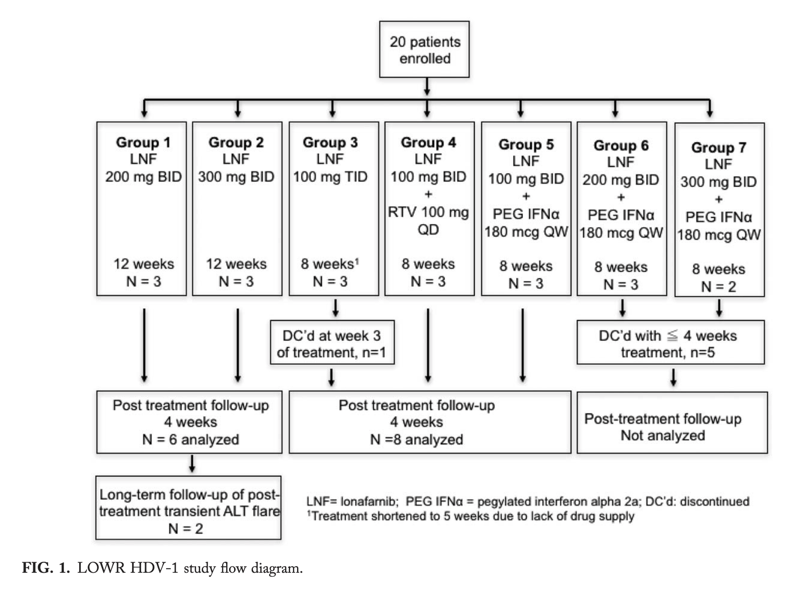
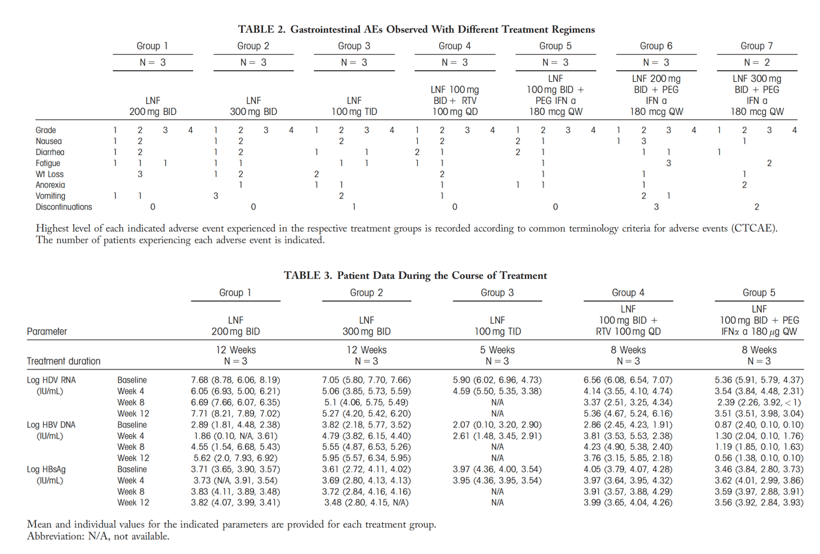
In a proof-of-concept (POC) study, the oral prenylation inhibitor, lonafarnib (LNF), decreased hepatitis D virus (HDV) RNA during 4 weeks of treatment. Here, we explored optimal LNF regimens. Fifteen patients (five groups; 3 per group) completed dosing as follows: (1) LNF 200 mg twice-daily (BID; 12 weeks); (2) LNF 300 mg BID (12 weeks); (3) LNF 100 mg thrice-daily (5 weeks); (4) LNF 100 mg BID + pegylated interferon alfa (PEG-IFNμ) 180 μg once-weekly (QW; 8 weeks); and (5) LNF 100 mg BID + ritonavir (RTV) 100 mg once-daily (QD; 8 weeks).
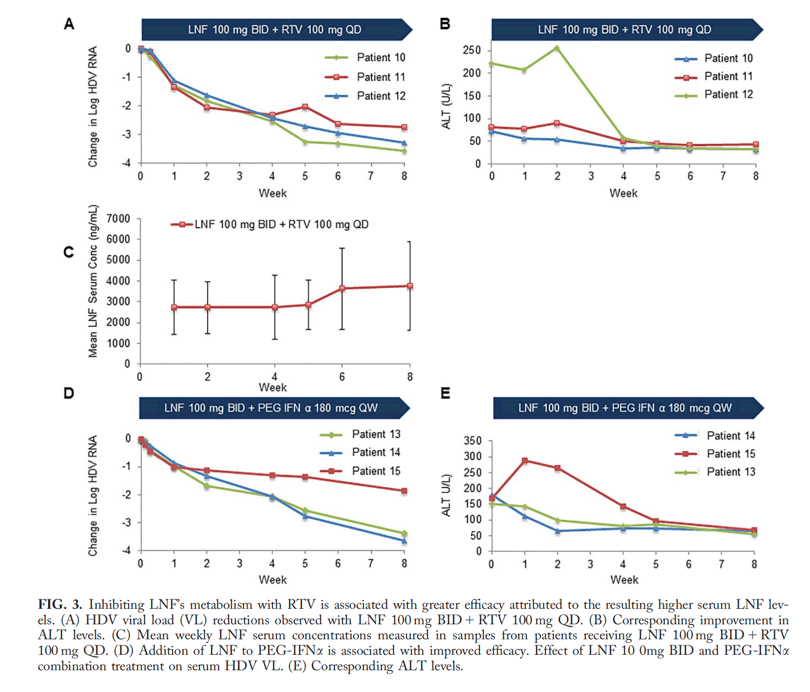
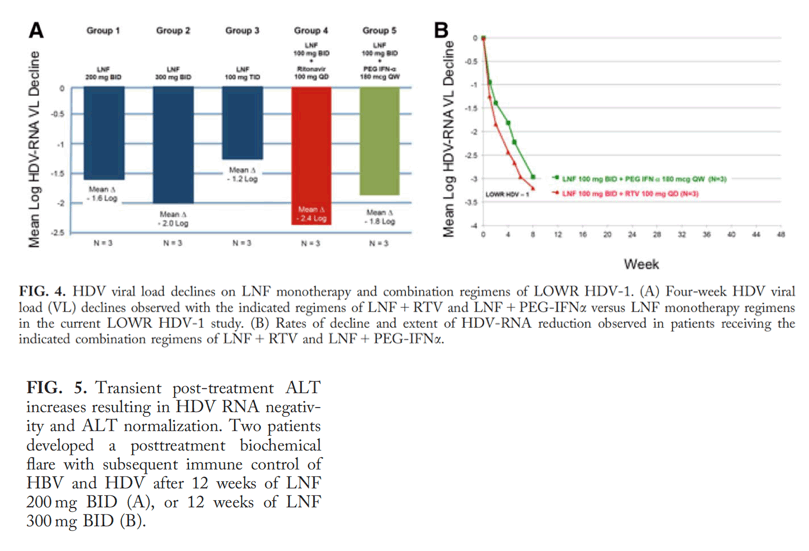
Tolerability and efficacy were assessed. Higher LNF monotherapy doses had greater decreases in HDV viral load than achieved in the original POC study. However, this was associated with increased gastrointestinal adverse events. Addition of RTV 100 mg QD to a LNF 100 mg BID regimen yielded better antiviral responses than LNF 300 mg BID monotherapy and with less side effects. A similar improvement was observed with LNF 100 mg BID + PEG-IFNμ 180 μg QW.
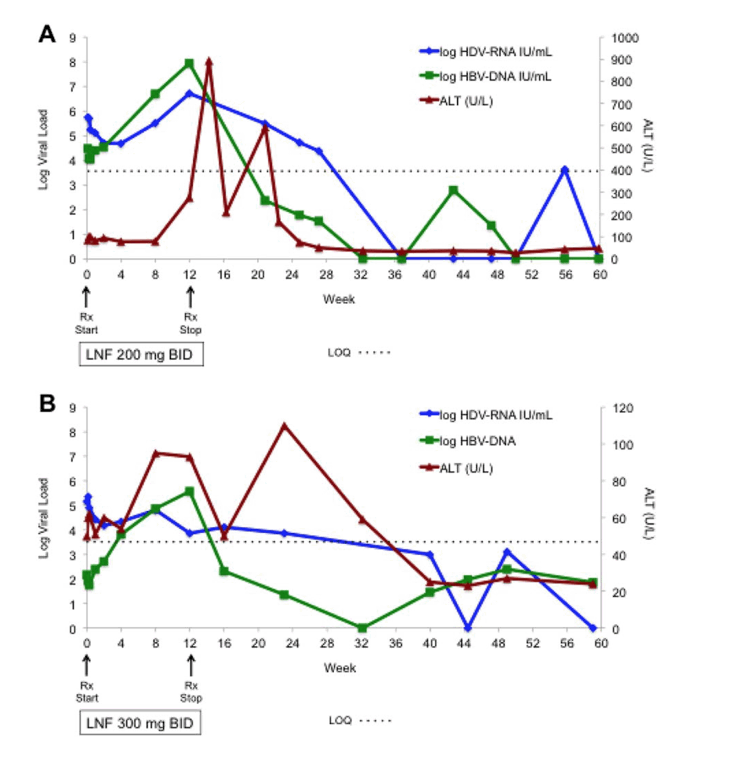
Two of 6 patients who received 12 weeks of LNF experienced transient posttreatment alanine aminotransferase (ALT) increases resulting in HDV-RNA negativity and ALT normalization.
Conclusion: The cytochrome P450 3A4 inhibitor, RTV, allows a lower LNF dose to be used while achieving higher levels of postabsorption LNF, yielding better antiviral responses and tolerability. In addition, combining LNF with PEG-IFNμ achieved more substantial and rapid HDV-RNA reduction, compared to historical responses with PEG-IFNμ alone. Twelve weeks of LNF can result in posttreatment HDV-RNA negativity in some patients, which we speculate results from restoring favorable immune responses. These results support further development of LNF with RTV boosting and exploration of the combination of LNF with PEG-IFN.
Introduction
Chronic delta hepatitis (CDH) infection leads to the most severe form of chronic viral hepatitis.1 The only treatment with proven efficacy consists of the use of conventional or pegylated interferon (IFN) alpha (PEG-IFNμ).2 Treatment response is observed in around 25%-40% after 1 year of treatment3-6 and extending treatment to 2 years does not appear to increase response rates.7-9 Still, there are data to suggest that IFN may need to be given for an extended duration of time,10, 11 which is consistent with in vitro studies that appear to lend support for longer treatment duration.12, 13 Viral kinetic studies also support the concept that CDH responds slower to IFN compared to hepatitis B (HBV) or hepatitis C virus (HCV).14
IFNμ must be given as subcutaneous injections and is associated with a plethora of side effects. For patients not responding to IFNμ, an alternative treatment does not exist. Hence, new treatment options are an urgent need in CDH. In this context, drugs targeting the hepatitis D virus (HDV) life cycle need to be explored. One such target is the virion assembly step in the hepatocyte cytoplasm, where the nascent HDV nucleoprotein complex is enveloped by hepatitis B surface antigen (HBsAg). This step involves the attachment of a 15-carbon prenyl group, farnesyl, to the large delta antigen, a reaction catalyzed by farnesyl transferase.15 Prenylation inhibitors have been shown to specifically abolish HDV-like particle production in vitro and in vivo.16, 17 Recently, the first human data have been reported.18 In that proof-of-concept (POC) study, the prenylation inhibitor, lonafarnib (LNF), dose dependently decreased HDV-RNA levels during 4 weeks of treatment, achieving 0.74 and 1.60 log reductions in HDV RNA with LNF 100 mg per oral (PO) twice-daily (BID) and LNF 200 mg PO BID, respectively. The aim of the current study was to explore additional dosing regimens capable of increasing the reduction in HDV viral load with LNF-based treatment, and assessing the safety and tolerability of LNF for up to 12 weeks.
VIRAL LOAD DETERMINATIONS
Quantitative HDV RNA was measured as described.18 This assay has a lower limit of quantification (LLOQ) of 70 IU/mL and lower limit of detection of 50 IU/mL, and the assay was standardized against the World Health Organization HDV-RNA standard. Serum HBV-DNA level was quantified by the CobasTaqMan HBV test (Roche Molecular Systems, Inc, Mannheim, Germany). Quantitative HDV-RNA viral load determinations for the long-term follow-up of the 2 patients who experienced transient posttreatment alanine aminotransferase (ALT) increases resulting in HDV-RNA negativity and ALT normalization were performed locally with an in-house PCR assay as described.20 This assay has an LLOQ of 6,170 IU/mL. HBsAg was quantified by the Architect HBsAg assay (Abbott Diagnostics, Germany) according to the manufacturer's instructions. Qualitative hepatitis serologies, including HBsAg, HBs antibody, hepatitis B e antigen (HBeAg), and HBe antibody (anti-HBe) were determined by a microparticle enzyme immunoassay method (Abbott Laboratories, North Chicago, IL), and anti-HDV was determined by an enzyme immunoassay (Abbott Laboratories).
|
|
| |
| |
|
|
|