| |
Treatment for hepatitis delta virus with
the prenylation inhibitor lonafarnib:
It's getting closer Editorial
|
| |
| |
Download the PDF here
24 January 2018
Hepatitis delta virus (HDV) is an incomplete RNA virus that is codependent on hepatitis B virus (HBV). It is currently estimated that approximately 15-20 million people worldwide are chronically infected with the virus.1
HDV is the most severe form of viral hepatitis. Rapid progression to cirrhosis occurs in the majority of infected individuals, and once cirrhosis has ensued, the risk for hepatocellular carcinoma increases 3-fold and for mortality 2-fold compared to patients with cirrhosis with HBV monoinfection.2, 3
Over the last three decades, tremendous progress made in HBV and HCV research has led to the development of highly efficacious medications capable of suppressing or eliminating these viruses. Unfortunately, similar advances in the management of HDV have not yet been accomplished. At present, there is no U.S. Food and Drug Administration–approved treatment for HDV. Pegylated interferon-alpha (PegIFN), the only available drug for HDV, shows at best, a 40% response rate following 48 weeks of therapy, but is poorly tolerated and is associated with high relapse rates.4 Treatment for HDV therefore presents a significant unmet medical need.
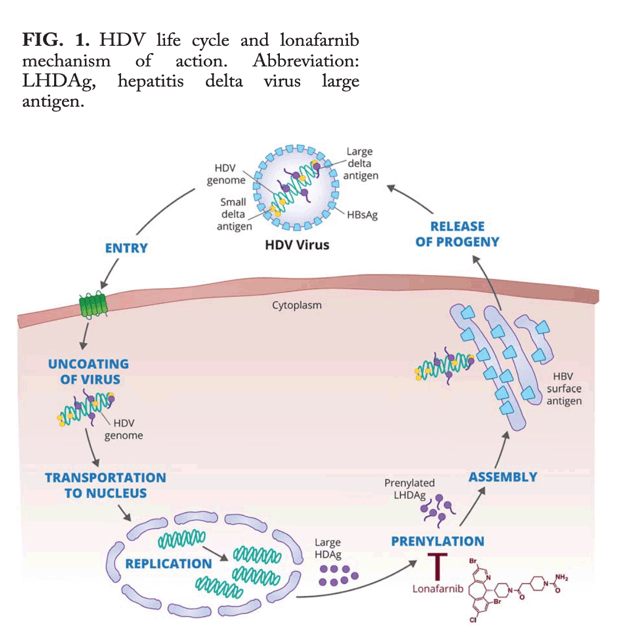
Development of new drugs for HDV is challenging, as unlike other hepatotrophic viruses, HDV encodes for only one protein and is completely dependent on host RNA polymerases for its replication. As a result there are relatively few HDV-specific targets to pursue for clinical development. At the current state of knowledge, it therefore seems that emergence of novel therapeutics for this disease will largely rely on the ability to intervene in nonreplicative steps of the HDV life cycle, such as viral entry and virion assembly.
One such possible strategy would involve disruption of hepatitis D antigen (HDAg) interaction with hepatitis B surface antigen (HBsAg). Prenylation is a posttranslational and site-specific modification of proteins that facilitates protein-protein and protein-membrane interactions. In a landmark report, more than two decades ago, Glenn et al. identified that prenylation of the carboxy terminus of the large HDAg was a necessary step for facilitating HDAg binding to HBsAg (Fig. 1) and subsequent HDV virion assembly.5 Farnesyltransferase inhibitors, were shown, in the following years, to inhibit HDV-like particles in in vitro cell lines and animal models.6, 7 Recently, in a proof-of-concept (POC) clinical trial, Koh et al. demonstrated that the farnesyltransferase inhibitor lonafarnib (LNF) administered at 100 mg BID (twice-daily) and 200 mg BID for 28 days led to statistically significant HDV viral load declines compared to placebo.8 Of note, nonresponse to LNF was not associated with development of resistant HDV mutations. Several additional and important observations were made during that study. First, a highly linear relationship was demonstrated between LNF serum concentration and HDV-RNA log decline, suggesting that using still higher concentrations of the drug may have an even greater antiviral effect. Second, the improved efficacy associated with the higher LNF dose came at the cost of more severe and frequent gastrointestinal (GI) side effects. Lastly, treatment discontinuation was followed by return of HDV viral load to pretreatment values in all study participants, implying that a longer treatment duration would be needed to achieve prolonged viral suppression or eradication.
The study by Yurdaydin et al., presented in this issue of Hepatology,9 further explores the utility of LNF for chronic HDV. The investigators tested different doses of LNF as monotherapy, and in combination with other drugs, for a duration of up to 12 weeks. As expected, higher doses of LNF monotherapy (200 and 300 mg BID) led to more profound viral suppression, but at the cost of more severe GI side effects. Two different strategies were explored for maintaining LNF's beneficial effect while improving its GI tolerability. The first was the addition of ritonavir (RTV), a cytochrome P450 3A4 (CYP3A4) inhibitor, to a low dose (100 mg BID) of LNF, a drug extensively metabolized by CYP3A4. This enabled less exposure of the drug to the GI tract, while achieving higher systemic exposure levels of LNF. Indeed, the LNF/RTV combination resulted in the best observed antiviral efficacy out of all study arms and a much-improved tolerability. Notably, the antiviral effect of high-dose LNF monotherapy reached its peak at Week 4 of treatment and then began to subside. This occurred in conjunction with declining LNF serum concentrations, which, as postulated by the investigators, resulted from impaired drug absorption attributed to diarrheal episodes. The antiviral suppressive effect of LNF/RTV combination, on the other hand, was maintained throughout the dosing period and was associated with LNF serum levels 4- to 5-fold higher than those reported in the POC study in patients treated with equivalent doses of LNF without RTV.
Another approach for optimizing LNF's antiviral effect was to combine a low dose (LNF 100 mg BID) with an immune-modulator such as PegIFN, a drug with proven activity against HDV. Indeed, this drug combination, too, showed comparable antiviral effect to high-dose LNF monotherapy, but with fewer side effects.
Overall, treatment with LNF in this study exhibits more rapid and profound drops in HDV-RNA levels than what has been historically observed in the largest trial to date of PegIFN alpha for HDV (HIDIT; Hep-Net International Delta Hepatitis Intervention Trial).4 With the paucity of therapeutic options available for treatment of HDV, these exciting results strongly support further development of LNF for future clinical use.
It is currently unclear whether elimination of HDV from the liver is possible or whether long-term sustained viral suppression akin to HBV therapy with nucleos(t)ide analogues is a more reasonable treatment goal. Achieving either goal will require the administration of therapy for extended periods of time with drugs that are reasonably tolerable and to which HDV does not develop resistance.
Results of the current study are encouraging in this sense because they lend additional support to Koh et al.'s observation regarding the high barrier to the development of resistance to LNF. Moving forward, this may allow exploration of even lower doses of LNF that could further improve the tolerability of this drug. Indeed, several studies are currently underway to explore treatment regimens with LNF doses as low as 25 mg BID combined with RTV with or without PegIFN.
As previously discussed, LNF does not inhibit HDV replication, thus allowing intracellular accumulation of HDV particles. The question remains whether HDV clearance from serum, even if temporary, could induce restoration of the immune response to HDV, leading, in turn, to viral eradication or long-term control. Yurdaydin et al. report that 2 of the 6 patients treated with LNF monotherapy for 12 weeks experienced posttreatment ALT flares associated with HDV decline to undetectable levels, that were then followed by ALT normalization and HDV levels fluctuating at very low or undetectable levels on long-term follow-up. This suggests that, conceptually, regaining immune control over the virus may be possible following LNF therapy, at least in a subset of patients. Larger-scale studies are needed to better characterize the effect of LNF treatment on HDV-specific immune responses.
After several decades with no major progress in the field, three novel agents for treatment of HDV are currently being evaluated at different stages of clinical development.10 The results of the study by Yurdaydin et al. suggest that LNF is likely to play an important role in this framework in the near future and may help offer long-awaited important treatment options for HDV patients.
Ohad Etzion M.D.
Institute of Gastroenterology and Liver Diseases
Soroka University Medical Center
Faculty of Health Sciences, Ben-Gurion University of the Negev
Beersheba, Israel
|
|
| |
| |
|
|
|