| |
HIV Capsid Lenacapavir Review Treatment & Prevention, PK, Mechanism, Resistance
|
| |
| |
Download the PDF here
Discovery of Novel Potent HIV Capsid Inhibitors with Long-Acting Potential
CROI 2017 Feb 14-17 Seattle, WA
Winston C Tse, John O Link, Andrew Mulato, Anita Niedziela-Majka, William Rowe, John R Somoza, Armando G Villasenor, Stephen R Yant, Jennifer R Zhang, Jim Zheng Gilead Sciences, Inc., Foster City, CA, USA
GS-6207: A Novel, Potent and Selective First-In-Class Inhibitor of HIV 1 Capsid Function Displays Nonclinical Pharmacokinetics Supporting Long Acting Potential in Humans
IAS: Long-acting subcutaneous lenacapavir dosed every 6 months as part of a combination regimen in treatment-naïve people with HIV: interim 16-week results of a randomized, open-label, phase 2 induction-maintenance study (CALIBRATE) - (07/20/21)
Interim Resistance Analysis of Long-Acting Lenacapavir in Treatment-Naïve People with HIV at 28 Weeks (CALIBRATE)
Efficacy and safety of long-acting subcutaneous lenacapavir in phase 2/3 in heavily treatment-experienced people with HIV: week 26 results (Capella study)

Dvory-Sobol, Hadas; Shaik, Naveed; Callebaut, Christian; Rhee, Martin S.
Current Opinion in HIV and AIDS Jan 2022
Lenacapavir interferes with multiple stages of the HIV life cycle (Fig. 1). By binding to two neighbouring subunits of the HIV capsid protein, lenacapavir interferes with their interactions essential for multiple phases of the viral replication cycle [4,5]. These include capsid-mediated nuclear uptake of preintegration complexes, virion production and proper capsid core formation. Virus produced in the presence of lenacapavir displays improperly shaped capsids that can enter new target cells but cannot replicate.
IN-VITRO POTENCY AND RESISTANCE PROFILE
In HIV-1 infected MT-4 cells, lenacapavir showed a mean half-maximum effective concentration (EC50) of 105 pmol/l, making it significantly more potent than many existing antiretroviral drugs [4]. Lenacapavir showed picomolar mean EC50 values in primary human CD4+ T cells (32 pmol/l) and macrophages (56 pmol/l) infected with HIV-1 and was broadly active against two HIV-2 isolates (885 pmol/l). In addition, lenacapavir displays similar antiviral activity against HIV-1 clinical isolates (A, A1, AE, AG, B, BF, C, D, G and H), with a mean EC50 value of 0.24 nmol/l and range from 0.15 to 0.36 nmol/l, in HEK293T cells [4,6].
In-vitro resistance selection assays in MT-2 cells and human peripheral blood mononuclear cells identified Q67H and N74D as the major resistance-associated mutations. Additional variants included L56I, M66I, K70N, Q67H/N74S and Q67H/T107N. These resistance-associated mutations, alone or in combination, confer reduced susceptibility to lenacapavir (six to >3200-fold resistance relative to wild-type). All but the low-level resistant variant Q67H (six-fold resistance to lenacapavir relative to wild-type virus) display reduced replication capacity in vitro. For example, M66I variant had only 1.5% replication capacity relative to wild type [6]. However, the clinical relevance of these findings is yet to be established.
Lenacapavir maintains potent antiviral activity against HIV-1 site-directed mutants and clinical isolates resistant to currently approved antiretrovirals in the following classes: nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), integrase strand transfer inhibitors (INSTIs) and protease inhibitors [7].
Lenacapavir is expected to be fully active regardless of prior treatment history because of its first-in-class nature. Among 1500 PWH, including treatment-naive (n = 500) or treatment-experienced (n = 1000; n = 500 with protease inhibitor experience and n = 500 without), none of the lenacapavir resistance mutations identified during in-vitro selection experiments was detected [8]. These data were confirmed in 182 treatment-naive individuals participating in CALIBRATE (see below), whose viruses had mean EC50 fold-change of 1.0 (range: 0.5-2.5) comparable to that of wild-type [6,9]. In addition, the antiviral activity of lenacapavir in clinical isolates is unaffected by the presence of naturally occurring Gag polymorphisms or Gag cleavage site mutations associated with protease inhibitor treatment and resistance to maturation inhibitors [7]. In contrast, those polymorphisms and mutations lead to reduced susceptibility to maturation inhibitors. These data suggest that lenacapavir is expected to have full activity regardless of prior treatment history.
PHARMACOKINETIC PROFILE
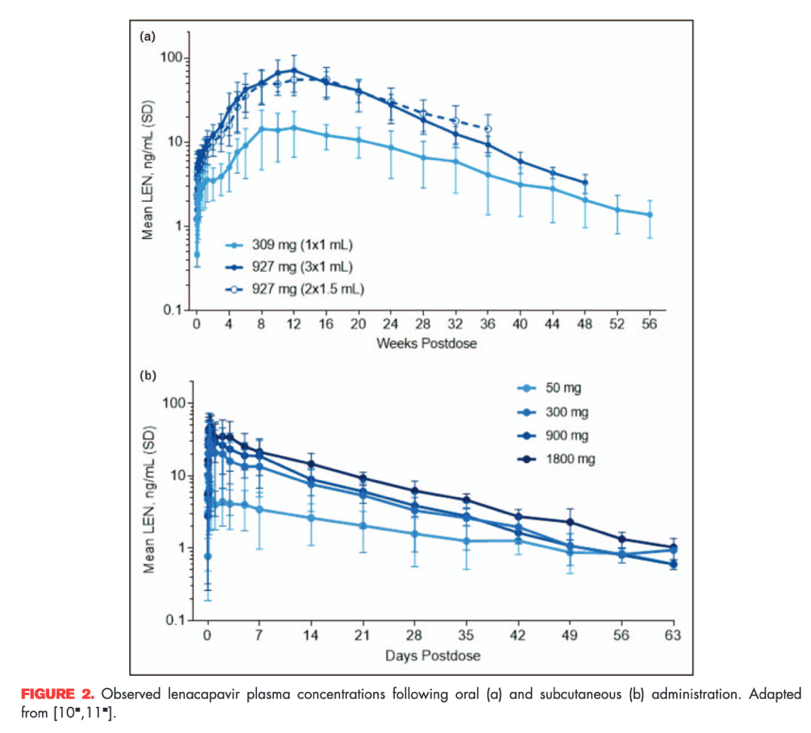
In a single-ascending clinical study in healthy participants, subcutaneous administration of lenacapavir had a slow, sustained release and dose-proportional increases in exposures up to 927 mg (Fig. 2). The slow release of lenacapavir from the administration site creates a complex absorption profile, with delayed and first-order absorption kinetics resulting in a Tmax of 77-84 days postdose and apparent t1/2 from 8 to 12 weeks [10].
After a single, 927 mg subcutaneous dose of lenacapavir, target plasma concentrations are sustained for at least 6 months, corresponding to a mean inhibitory quotient of at least 6 (i.e. six-fold higher than the protein-adjusted EC95). However, a slow initial rise after administration of subcutaneous lenacapavir in the first few weeks was noted, leading to consideration of initial pharmacokinetic loading with oral lenacapavir.
For oral tablets, single-dose administration in the range from 50 to 1800 mg showed increased exposure with higher doses, but this increase is not dose proportional (Fig. 2) [11]. Oral lenacapavir has a t1/2 of nearly 10-12 days, and absorption is not affected by the presence of food. In ongoing clinical studies, oral lenacapavir is being used for initial pharmacokinetic loading prior to initiating subcutaneous administration every 6 months (Fig. 3). In addition, oral lenacapavir can be administered daily or weekly in combination with other antiretroviral agents, which can facilitate development of multiple lenacapavir-containing regimens with dosing intervals ranging from daily to weekly or even up to every 6 months.
DRUG-DRUG INTERACTIONS
The potential for drug-drug interactions with lenacapavir were evaluated in a clinical study [12]. Inhibitors of CYP3A and CYP3A/P-gp increased lenacapavir exposures, but not to a clinically relevant extent, supporting their coadministration with lenacapavir (e.g. cobicistat). Strong inducers of CYP3A/P-gp/UGT1A1 (e.g. rifampin) reduced lenacapavir exposures significantly, and their coadministration is contraindicated. Lenacapavir is a moderate inhibitor of CYP3A and caution is required for coadministration with sensitive CYP3A substrates with a narrow therapeutic window. There is no restriction on co-administration of lenacapavir with BCRP, P-gp and OATP substrates or with acid-reducing agents. Most of the commonly used antiretroviral agents have no clinically relevant drug-drug interaction potential with lenacapvir. However, some are not recommended due to strong inhibition potential (e.g. boosted atazanavir, which inhibits all three of CYP3A/P-gp/UGT1A1) or moderate induction potential (e.g. efavirenz, nevirapine or boosted tipranavir). Sex-affirming hormones and oral contraceptives are not expected to have clinically relevant drug-drug interactions with lenacapavir.
LENACAPAVIR FOR TREATMENT OF PERSONS WITH HIV-1: CLINICAL STUDIES
Proof of concept
In PWH not taking antiretroviral therapy, lenacapavir demonstrated potent antiviral activity, with declines of up to 2.3 log10 copies/ml in 10 days in a proof-of-concept, dose-ranging study of single subcutaneous doses (20, 50, 150, 450 or 750 mg; n = 6/cohort) [13]. The Emax on Day 10 postdose was predicted to be a 2.1 log10 decline in HIV-1 RNA, and the mean lenacapavir concentration of at least 4.4 ng/ml (inhibitory quotient ≥1.1) was predicted to provide near maximal antiviral activity.
CAPELLA study (clinicaltrials.gov identifier: NCT04150068)
Thirty-six HTE PWH who were failing their existing regimen were randomly assigned (2 : 1) to lenacapavir or placebo in a double-blind manner (Fig. 4) [14,15]. Participants had HIV-1 RNA at least 400 c/ml and were resistant to at least two agents from at least three of the four major antiretroviral classes. For the first 2 weeks, in addition to receiving their existing antiretroviral regimen, 24 participants received oral lenacapavir (600 mg on Days 1 and 2 and 300 mg on Day 8), and 12 received placebo. Thus, these first 14 days were considered a period of functional monotherapy. At Day 15, all participants initiated an investigator-selected optimized background regimen. Those initially assigned to oral lenacapavir started subcutaneous lenacapavir (927 mg) every 6 months, while those on placebo started a lenacapavir 2-week oral lead-in, followed by subcutaneous, 6-month dosing. Subsequently, an additional nonrandomized cohort of 36 participants received 14 weeks of oral lenacapavir followed by subcutaneous lenacapavir every 6 months.
Following initiation of lenacapavir, rapid reductions in HIV-1 RNA were observed among the 36 randomized patients; at the end of the first 14 days (functional monotherapy), 88% (21/24) receiving lenacapvir had a reduction in HIV-1 RNA of at least 0.5 log10 copies/ml compared with 17% (2/12) receiving placebo. At Week 26, 81% of randomized participants (29/36) had HIV-1 RNA less than 50 copies/ml. Four randomized participants had emergent lenacapavir resistance. Lenacapavir was well tolerated. No serious adverse events related to study drug occurred, and there were no discontinuations due to adverse events.
CALIBRATE study (clinicaltrials.gov identifier: NCT04143594)
Lenacapavir is also being evaluated in an open-label, Phase 2, active-controlled, induction-maintenance study of treatment-naive PWH with CD4+ cell counts at least 200 cells/μl (Fig. 4). Participants were randomized (2 : 2:2 : 1) to one of four treatment groups [16]. The first and second groups received lenacapavir (given initially orally for loading/lead-in, then subcutaneously) with oral daily emtricitabine/tenofovir alafenamide, prior to switching from emtricitabine/tenofovir alafenamide to oral daily tenofovir alafenamide (Group 1) or bictegravir (Group 2). The third group received oral daily lenacapavir with emtricitabine/tenofovir alafenamide throughout the study. The fourth group, a control arm, received oral daily bictegravir/emtricitabine/tenofovir alafenamide.
Lenacapavir, administered subcutaneously or orally, in combination with emtricitabine/tenofovir alafenamide led to high rates of viral suppression by Week 28 (94%; n = 147/157). One participant receiving subcutaneous lenacapavir and emtricitabine/tenofovir alafenamide had emergent resistance to lenacapavir. Lenacapavir was well tolerated. No serious adverse events or Grade 4 adverse events were reported as related to study drug. Two participants discontinued due to adverse events (both due to Grade 1 injection site induration).
Lenacapavir for prevention of HIV-1 in persons at risk: preclinical studies
GS-CA1, a close lenacapavir analogue active against both HIV-1 and SIV, has been evaluated as a long-acting PrEP agent in a macaque rectal SHIV challenge model [17]. Uninfected Indian rhesus macaques received a single subcutaneous injection of vehicle control (placebo), 150 mg/kg (low dose) or 300 mg/kg (high dose) GS-CA1, followed by weekly escalating titre SHIV challenges starting 1 week postdosing. After a total of 15 challenges, eight out of eight placebo-exposed animals became infected, whereas two out of eight animals receiving low-dose GS-CA1 and five out of eight receiving high-dose GS-CA1 remained protected. The median time to infection was 7.5 weeks in the placebo group, 16 weeks in the low-dose GS-CA1 group and not reached due to insufficient number of infections in the high-dose group. Relative to the placebo group, the low-dose and high-dose treatment groups demonstrated an 86% (P = 0.0061) and 96% (P = 0.0002) reduction in risk of infection, respectively, as determined by Cox regression analysis. Notably, on the basis of a 2-week infection-to-detection window, treatment group infections occurred only after plasma GS-CA1 concentrations fell below 2X paEC95.
Future directions
As a first in class capsid inhibitor, lenacapavir demonstrated potent antiviral activity in individuals with HIV-1, prophylactic activity in macaques and high efficacy in combination with other antiretrovirals for individuals at two ends of the treatment spectrum: those initiating therapy for the first time and those who are heavily treatment experienced with multidrug resistance. Future studies will investigate ways to translate the unique properties of lenacapavir, such as its novel mechanism of action with no preexisting resistance, flexible route of administration (oral or injectable) and dosing interval (daily, weekly or up to every 6 months), into clinical benefit. Lenacapavir has the potential to address the diverse needs of individuals with HIV-1, who have varied treatment histories, prior resistance, status of viremia and preferences for daily or long-acting therapy, as well as those at risk for HIV-1.
Long-acting regimens with infrequent dosing, for example injectable up to every 6 months or oral weekly, have a potential role in addressing challenges with adherence to oral daily therapy and treatment fatigue associated with it. Subcutaneous lenacapavir administered by healthcare providers every 6 months coincides with the minimum clinical visit schedule and does not pose additional treatment burden for many PWH. An oral weekly regimen could benefit individuals who prefer an oral regimen but one that can be taken less frequently than daily. There are ongoing efforts to develop a long-acting partner agent to be combined with lenacapavir for a complete long-acting injectable or oral weekly regimen. Currently, lenacapavir is being evaluated in combination with islatravir, a nucleoside reverse transcriptase translocation inhibitor (NRTTI) as an oral weekly regimen in virologically suppressed PWH (ClinicalTrials.gov Identifier: NCT05052996). An injectable regimen of islatravir and lenacapavir with a longer dosing interval is also being developed.
Subcutaneous lenacapavir administered every 6 months is being evaluated as PrEP in two Phase 3 studies: PURPOSE-1 (ClinicalTrials.gov Identifier: NCT04994509) in adolescent girls and young women at risk of HIV-1 infection and PURPOSE-2 (ClinicalTrials.gov Identifier: NCT04925752) in cisgender men, transgender women, transgender men and sex nonbinary people at least 16 years of age at risk for HIV-1 infection.
Lenacapavir is a first-in-class capsid inhibitor with long-acting properties. It has the potential to be developed as part of an injectable regimen with an up to 6-month dosing interval or an oral regimen with an up to weekly dosing interval, providing additional treatment options to address the diverse needs of persons with HIV-1 and for those who would benefit from PrEP.
|
|
| |
| |
|
|
|