| |
Evolocumab (PCSK9 Inhibitor for Heart Disease) in HIV-Infected Patients With Dyslipidemia: Primary Results of the Randomized, Double-Blind BEIJERINCK Study
|
| |
| |
Download the PDF here
Franck Boccara, MD, PHD,a Princy N. Kumar, MD,b Bruno Caramelli, MD, PHD,c Alexandra Calmy, MD, FMH, PHD,d
J. Antonio G. López, MD,e Sarah Bray, PHD,e Marcoli Cyrille, MD,e Robert S. Rosenson, MD,f for the BEIJERINCK Investigators
J Am Coll Cardiol. 2020 May
Abstract
Background
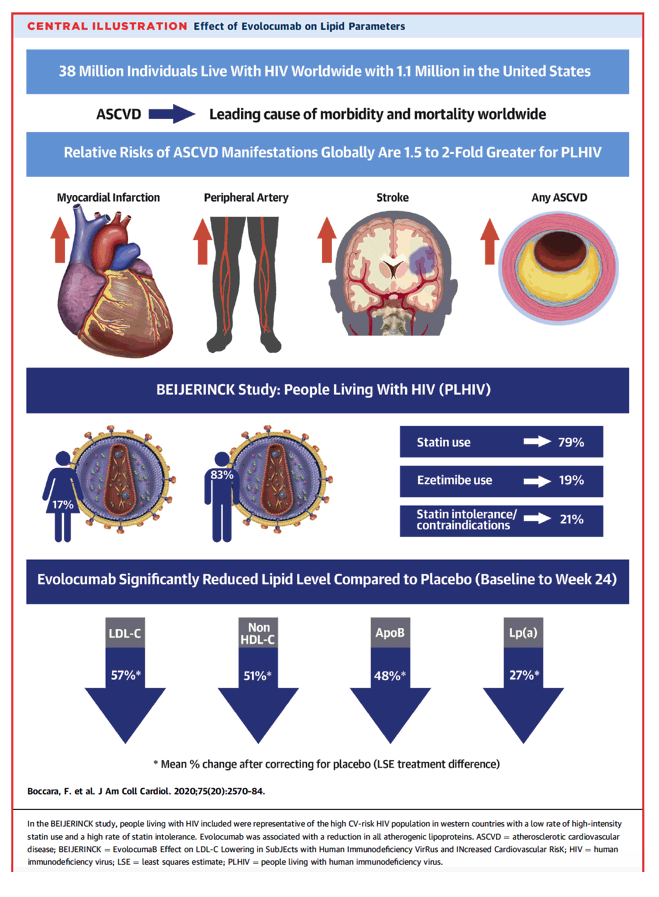
People living with human immunodeficiency virus (PLHIV) are at increased risk of atherosclerotic cardiovascular disease (ASCVD) and are prone to statin-related adverse events from drug-drug interactions with certain antiretroviral regimens.
Objectives
This study sought to evaluate the efficacy and safety of evolocumab in dyslipidemic PLHIV.
Methods
BEIJERINCK (EvolocumaB Effect on LDL-C Lowering in SubJEcts with Human Immunodeficiency VirRus and INcreased Cardiovascular RisK) is a randomized, double-blind, multinational trial comparing monthly subcutaneous evolocumab 420 mg with placebo in PLHIV with hypercholesterolemia/mixed dyslipidemia taking maximally-tolerated statin therapy. The primary endpoint was the percent change (baseline to week 24) in low-density lipoprotein cholesterol (LDL-C); secondary endpoints included achievement of LDL-C <70 mg/dl and percent change in other plasma lipid and lipoprotein levels. Treatment-emergent adverse events were also examined.
Results
A total of 464 patients were analyzed (mean age of 56.4 years, 82.5% male, mean duration with HIV of 17.4 years). ASCVD was documented in 35.6% of patients, and statin intolerance/contraindications to statin use were present in 20.7% of patients. Evolocumab reduced LDL-C by 56.9% (95% confidence interval: 61.6% to 52.3%) from baseline to week 24 versus placebo. An LDL-C level of <70 mg/dl was achieved in 73.3% of patients in the evolocumab group versus 7.9% in the placebo group. Evolocumab also significantly reduced other atherogenic lipid levels, including non-high-density lipoprotein cholesterol, apolipoprotein B, and lipoprotein(a) (all p < 0.0001). Evolocumab was well tolerated, and treatment-emergent adverse events patient incidence was similar among evolocumab and placebo groups.
Conclusions
Evolocumab was safe and significantly reduced lipid levels in dyslipidemic PLHIV on maximally-tolerated statin therapy. Evolocumab is an effective therapy for lowering atherogenic lipoproteins in PLHIV with high cardiovascular risk. (Safety, Tolerability & Efficacy on LDL-C of Evolocumab in Subjects With HIV & Hyperlipidemia/Mixed Dyslipidemia; NCT02833844)
|
|
| |
| |
|
|
|