| |
PCSK9 (Repatha) in HIV+, Affect on Plaque
|
| |
| |
Evolocumab (PCSK9 Inhibitor for Heart Disease) in HIV-Infected Patients With Dyslipidemia: Primary Results of the Randomized, Double-Blind BEIJERINCK Study - (01/27/22)
Evolocumab induced plaque regression in a greater percentage of patients than placebo (64.3% vs 47.3% - Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients - The GLAGOV Randomized Clinical Trial
Atherosclerosis, a systemic disease,1 forms the substrate for the majority of ischemic cardiovascular events. Considerable evidence from autopsy and vascular imaging studies relates the burden of atherosclerosis to its clinical sequelae.2-8 Intravascular ultrasound (IVUS) has evolved as an imaging modality that generates high-resolution, precise volumetric quantification of epicardial coronary atherosclerosis. By measuring the change in atheroma volume over time, IVUS can evaluate the potential anti-atherosclerotic efficacy of interventions on plaquedevelopment.9
This trial also evaluated the percentage of patients demonstrating regression of coronary atherosclerosis, defined as any change in PAV or TAV less than zero. Using this definition, for the primary end point, PAV, approximately 47% of patients in the placebo group experienced regression, compared with 64% of the treatment group receiving the combination of a statin and PCSK9 inhibitor (between-group difference, 17.0%; P < .001). Similar results were observed for TAV, with more patients achieving regression with combination It is also the first, to our knowledge, to demonstrate a reduction in atherosclerotic disease progression by IVUS for a nonstatin LDL-C-lowering therapy.
to our knowledge, no trials to date have explored whether LDL-C lowering with a PCSK9 inhibitor reduces the rate of progression of coronary atherosclerosis, and no data exist assessing whether achieving very low LDL-C levels via combination therapy results in incremental benefits in reducing disease progression compared with statins alone. The GLAGOV (Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound) trial was designed to assess whether PCSK9 inhibition reduces progression of atherosclerosis as measured by IVUS.
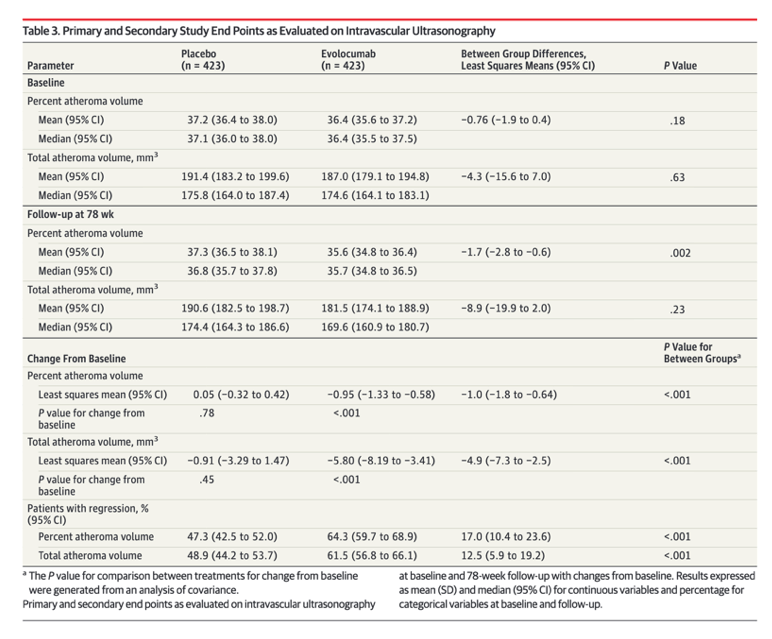
Primary and Secondary IVUS End Points
Changes in IVUS measures of plaque burden are summarized in Table 3. The primary efficacy measure, PAV, did not change in the placebo group (0.05%, P = .78 compared with baseline) and decreased by 0.95% in the evolocumab group (P < .001 compared with baseline; between-group difference, -1.0% [95% CI, -1.8% to -0.64%]; P < .001). The secondary efficacy measure, TAV, did not change in the placebo group (-0.9 mm3, P = .45 compared with baseline) and decreased by 5.8 mm3 in the evolocumab group (P < .001 compared with baseline; between-group difference, -4.9 mm3 [95% CI, -7.3 to -2.5]; P < .001). More evolocumab-treated patients exhibited PAV regression (64.3% vs 47.3%, P < .001) and TAV regression (61.5% vs 48.9%, P < .001)
OBJECTIVE To determine the effects of PCSK9 inhibition with evolocumab on progression of
coronary atherosclerosis in statin-treated patients.
Participants with angiographic coronary disease were randomized to receive monthly evolocumab (420 mg) (n = 484) or placebo (n = 484) via subcutaneous injection for
76 weeks, in addition to statins.
MAIN OUTCOMES AND MEASURES -
The primary efficacy measure was the nominal change in
percent atheroma volume (PAV) from baseline to week 78, measured by serial intravascular
ultrasonography (IVUS) imaging. Secondary efficacy measures were nominal change in
normalized total atheroma volume (TAV) and percentage of patients demonstrating plaque
regression. Safety and tolerability were also evaluated.
The primary efficacy parameter, PAV, increased 0.05% with placebo and decreased 0.95% with
evolocumab (difference, -1.0% [95% CI, -1.8% to -0.64%]; P < .001). The secondary efficacy
parameter, normalized TAV, decreased 0.9 mm3 with placebo and 5.8 mm3 with evolocumab
(difference, -4.9 mm3 [95% CI, -7.3 to -2.5]; P < .001). Evolocumab induced plaque
regression in a greater percentage of patients than placebo (64.3% vs 47.3%; difference,
17.0% [95% CI, 10.4% to 23.6%]; P < .001 for PAV and 61.5% vs 48.9%; difference, 12.5%
[95% CI, 5.9% to 19.2%]; P < .001 for TAV).
CONCLUSIONS AND RELEVANCE -
Among patients with angiographic coronary disease treated with statins, addition of evolocumab, compared with placebo, resulted in a greater decrease in PAV after 76 weeks of treatment. Further studies are needed to assess the effects of PCSK9 inhibition on clinical outcomes.
|
|
| |
| |
|
|
|