| |
PCSK9 - A Journey to Cardiovascular Outcomes Editorial
|
| |
| |
Download the PDF PCSK9 - A Journey to Cardiovascular Outcomes
Download the PDF Alirocumab and Cardiovascular Outcomes after Acute
Coronary Syndrome
Download the PDF Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events
Some patients do not reach target low-density lipoprotein (LDL) cholesterol levels despite receiving treatment with high-intensity statins. Monoclonal antibodies that inhibit proprotein convertase subtilisin–kexin type 9 (PCSK9) have emerged as an effective treatment to address this gap and can lower LDL cholesterol by approximately 60% among patients who are already receiving standard therapy.1,2
PCSK9 is secreted by the liver and is found in 1 in every 500 LDL particles in the circulation. When these PCSK9-associated particles bind to the LDL receptor, the complex is internalized and the receptor is targeted for degradation rather than recycling back to the cell surface.3 The role of PCSK9 in lipid metabolism was established in 2003 with the discovery of rare gain-of-function mutations that cause autosomal dominant hypercholesterolemia.4 This discovery was followed by studies that showed that loss-of-function PCSK9 mutations lower LDL cholesterol levels and protect against cardiovascular disease.5 PCSK9 inhibitors mimic this effect; their mechanism of action involves both accelerated removal and decreased production of LDL particles.6,7
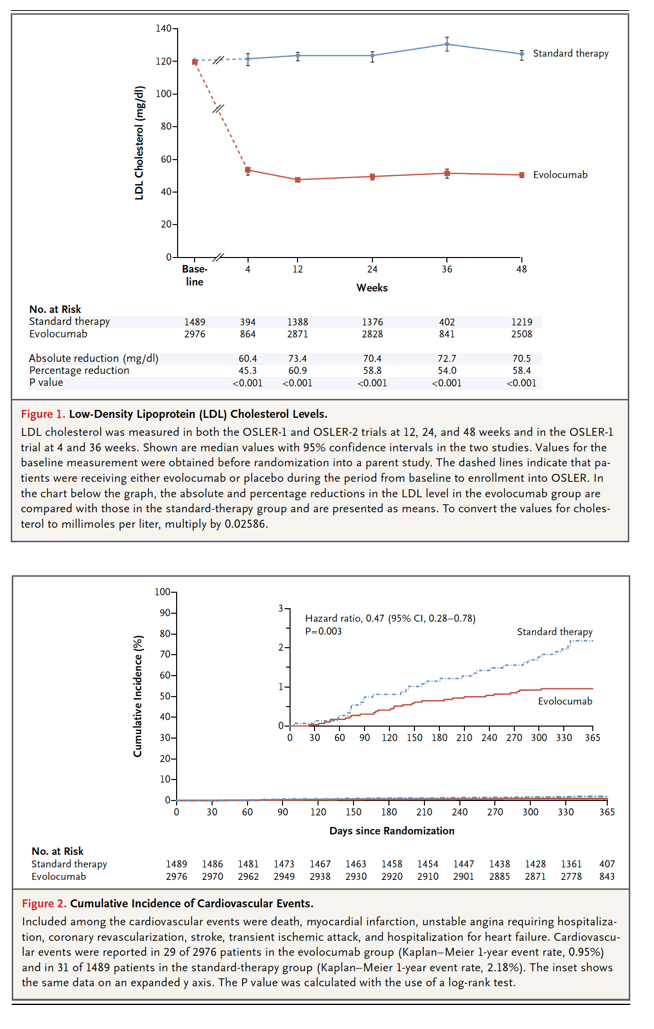
In 2015, the efficacy of alirocumab and of evolocumab - two fully human monoclonal antibodies that target PCSK9 - in reducing LDL cholesterol levels was confirmed. In the ODYSSEY LONG TERM trial (Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy), which involved 2341 patients at high risk for cardiovascular events who had an LDL cholesterol level of 70 mg per deciliter (1.8 mmol per liter) or more, the addition of alirocumab (150 mg subcutaneously every 2 weeks) to statin therapy resulted in an LDL cholesterol level that was 62% lower than the level in the placebo group at week 24.1 In the OSLER-1 and OSLER-2 trials (Open-Label Study of Long-Term Evaluation against LDL Cholesterol), which included 4465 patients overall, the addition of evolocumab (140 mg subcutaneously every 2 weeks or 420 mg monthly for a median of 11.1 months) to standard therapy resulted in an LDL cholesterol level that was 61% lower than the level in the standard-therapy group.2 In post hoc and exploratory analyses, both the ODYSSEY LONG TERM trial and the OSLER trials showed a risk of cardiovascular events that was approximately 50% lower among the patients who received the PCSK9 inhibitor than among those who received standard treatment over the short-term course of the trials; however, the event rates were low and the trials were inadequately powered to assess cardiovascular outcomes.1,2
In this issue of the Journal, Schwartz et al. report the results of ODYSSEY OUTCOMES, an international, multicenter, randomized, double-blind, placebo-controlled trial.8 A total of 18,924 patients who had had an acute coronary syndrome 1 to 12 months earlier and were receiving statin therapy at a high-intensity dose or at the maximum tolerated dose were randomly assigned to receive alirocumab subcutaneously at a dose of 75 mg or matching placebo every 2 weeks and were followed for a median of 2.8 years.
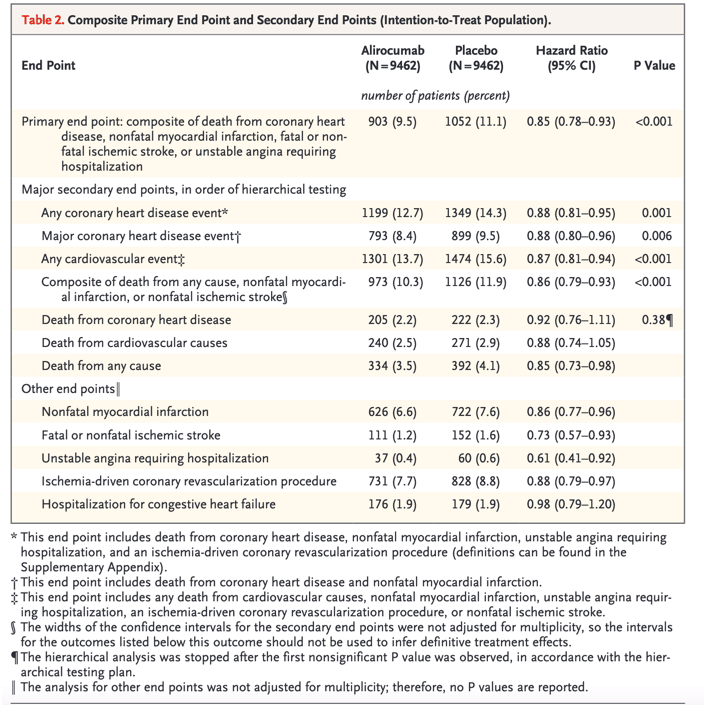
The primary end point of the ODYSSEY OUTCOMES trial was a composite of death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization. A composite primary end-point event occurred in 903 patients (9.5%) in the alirocumab group and in 1052 patients (11.1%) in the placebo group, resulting in a hazard ratio of 0.85 (95% confidence interval [CI], 0.78 to 0.93). However, no significant differences between the trial groups were observed with respect to the risks of the secondary end points of death from coronary heart disease and death from cardiovascular causes. The investigators calculated that 49 patients would need to be treated for 4 years to prevent the occurrence of one primary end-point event.
In the ODYSSEY OUTCOMES trial, the lowering of LDL cholesterol with alirocumab, although sustained, was somewhat less pronounced than it was in the ODYSSEY LONG TERM trial1 and may reflect discontinuation of treatment or decreases in the dose. The ODYSSEY OUTCOMES trial was not designed to explore the safety of sustained, extremely low LDL cholesterol levels, so doses of alirocumab were adjusted to reach an LDL cholesterol level of 25 to 50 mg per deciliter (0.6 to 1.3 mmol per liter). Among the 9462 patients who were receiving alirocumab, 730 patients (7.7%) were switched to placebo under blinded conditions because they had two consecutive measurements of LDL cholesterol that were below 15 mg per deciliter (0.39 mmol per liter) while they were receiving the 75-mg dose; for 2615 patients (27.6%), the dose of alirocumab was increased to 150 mg under blinded conditions because they had an LDL cholesterol level of 50 mg per deciliter or more. The incidence of adverse events was similar in the alirocumab group and the placebo group, except for local injection-site reactions, which were more common with alirocumab (3.8% vs. 2.1%).
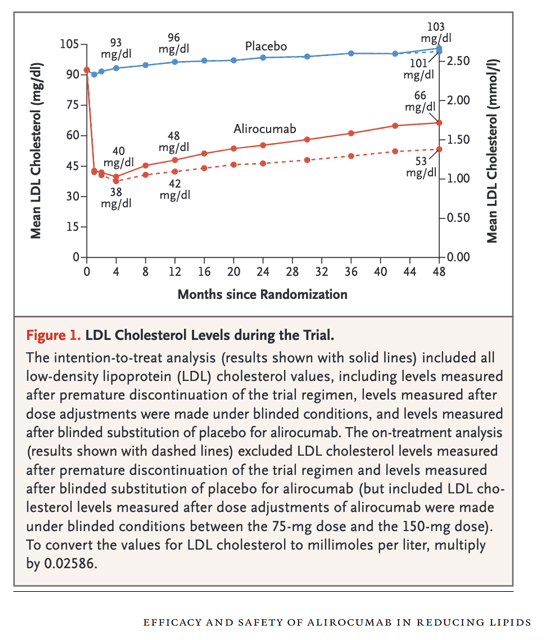
The FOURIER trial (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk), which included 27,564 patients who had stable atherosclerotic cardiovascular disease and were receiving statin therapy, assessed the effects of the PCSK9 inhibitor evolocumab on cardiovascular outcomes.9 The risk of the primary end point (a composite of death from cardiovascular causes, myocardial infarction, stroke, unstable angina requiring hospitalization, or coronary revascularization) was lower among those who were treated with evolocumab than among those who received placebo (hazard ratio, 0.85; 95% CI, 0.79 to 0.92). The ODYSSEY OUTCOMES trial and the FOURIER trial showed similarly low rates of major adverse cardiovascular events among the patients who were treated with the PCSK9 inhibitors. However, although the ODYSSEY OUTCOMES trial showed a lower risk of death from any cause with the PCSK9 inhibitor than with placebo, the FOURIER trial did not, and neither trial showed a significantly lower risk of death from cardiovascular causes.8,9
The results of the ODYSSEY OUTCOMES trial will change clinical practice. The use of alirocumab to target an LDL cholesterol level of 25 to 50 mg per deciliter after an acute coronary syndrome resulted in a lower risk of major adverse cardiac events than placebo. Patients who had a baseline LDL cholesterol level of 100 mg per deciliter (2.6 mmol per liter) or more seemed to derive more benefit. However, the high cost of monoclonal antibodies to PCSK9 (even after recent substantial price reductions in the United States) obligates careful consideration of the type of high-risk patient who would most benefit from their clinical use.10
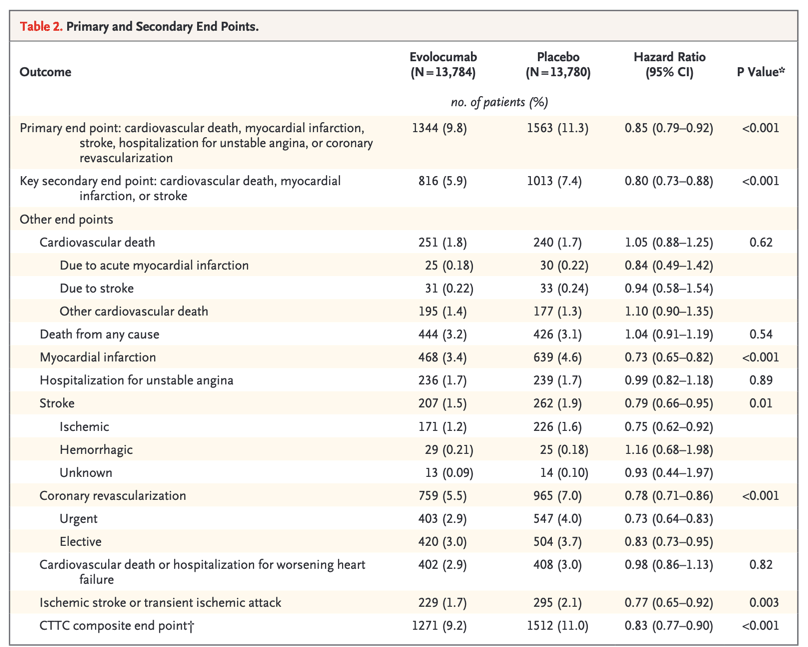
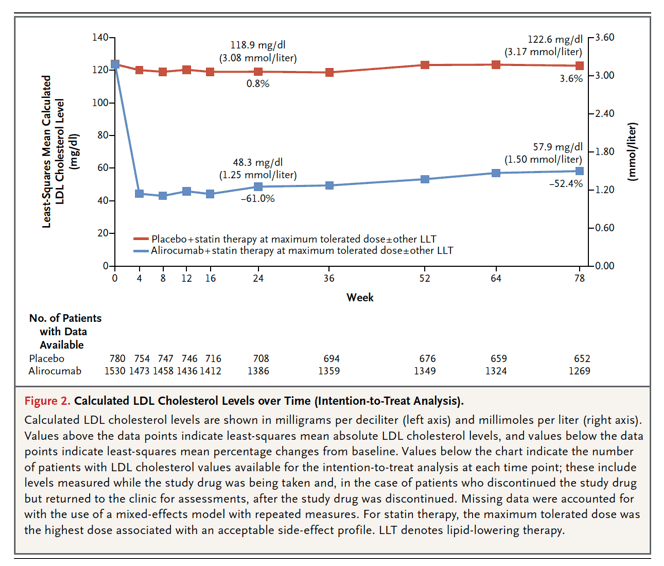
Lowering of LDL cholesterol levels with alirocumab was sustained but to a lesser extent than that reported in previous trials that had a shorter duration.9 The increase in LDL cholesterol over time in the intention-to-treat analysis reflects premature discontinuation of treatment, dose reduction or substitution of placebo for alirocumab under blinded conditions, and attenuation of the intensity of statin treatment. The last factor probably also contributed to the rise in LDL cholesterol observed in the placebo group, in the on-treatment analysis in the alirocumab group, and in previous trials involving patients who had an acute coronary syndrome.5,21 Antidrug antibodies were detected in few patients and have been shown not to influence the lipid-lowering efficacy of alirocumab.22
|
|
| |
| |
|
|
|