| |
Gilead Announces Clinical Hold on Studies Evaluating Injectable
Lenacapavir for HIV Treatment and Prevention Due to Vial Quality Concerns
|
| |
| |
-- Pause Due to Concerns About Compatibility of the Vial Type with the Drug Solution --
December 21, 2021 07:12 PM Eastern Standard Time
FOSTER CITY, Calif.--(BUSINESS WIRE)--Gilead Sciences, Inc. (Nasdaq: GILD) today announced that the U.S. Food and Drug Administration (FDA) has placed a clinical hold on the use of injectable lenacapavir in borosilicate vials in all ongoing clinical studies for HIV treatment and HIV pre-exposure prophylaxis (PrEP). The FDA's clinical hold is due to emerging concerns about the compatibility of vials made of borosilicate glass with lenacapavir solution, which could potentially lead to the formation of sub-visible glass particles in the solution of lenacapavir. Dosing of oral formulations of lenacapavir will continue. The company remains confident about the future potential of lenacapavir and is committed to resolving this vial quality issue.
During the clinical hold, screening and enrollment of study participants and the dosing of injectable lenacapavir will not be permitted across all lenacapavir studies. All other study activities, including the monitoring of participants and the dosing of participants in comparator arms, will continue according to the relevant study protocol.
"We are committed to working diligently with FDA to resolve this glass vial compatibility quality issue and resume injectable lenacapavir dosing in the affected studies in a timely fashion," said Merdad Parsey, MD, PhD, Chief Medical Officer, Gilead Sciences.
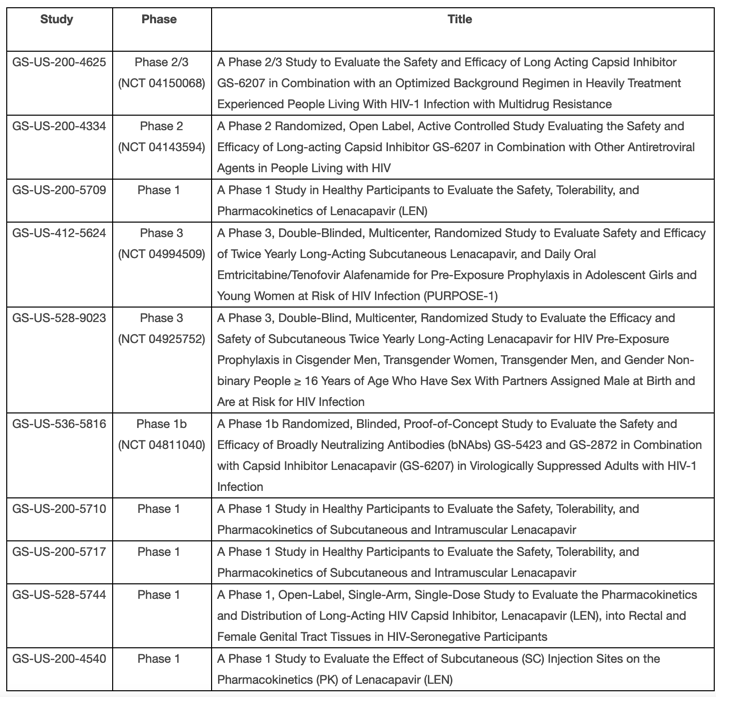
The following studies have been placed on clinical hold:
Lenacapavir is an investigational compound and is not approved by any regulatory authority for any use and its safety and efficacy are not known. There is no cure for HIV or AIDS.
About Lenacapavir
Lenacapavir is Gilead's potential first-in-class, investigational long-acting HIV-1 capsid inhibitor in development for the treatment and prevention of HIV-1 infection. Lenacapavir's multi-stage mechanism of action is distinguishable from currently approved classes of antiviral agents and is designed to provide a new avenue for the development of long-acting therapy options for people living with or at risk for HIV-1. While most antivirals act on just one stage of viral replication, lenacapavir is designed to inhibit HIV-1 at multiple stages of its lifecycle.
About Gilead Sciences
Gilead Sciences, Inc. is a biopharmaceutical company that has pursued and achieved breakthroughs in medicine for more than three decades, with the goal of creating a healthier world for all people. The company is committed to advancing innovative medicines to prevent and treat life-threatening diseases, including HIV, viral hepatitis and cancer.
For more than 30 years, Gilead has been a leading innovator in the field of HIV, driving advances in treatment, prevention and cure research. Gilead researchers have developed 11 HIV medications, including the first single tablet regimen to treat HIV and the first antiretroviral for pre-exposure prophylaxis (PrEP) to reduce the risk of acquiring HIV infection. These advances in medical research have helped to transform HIV into a preventable, chronic condition for millions of people.
Gilead is committed to continued scientific innovation to provide solutions for the evolving needs of people affected by HIV around the world. Through partnerships and collaborations, the company also aims to improve education, expand access and address barriers to care, with the goal of ending the HIV epidemic for everyone, everywhere.
Gilead operates in more than 35 countries worldwide, with headquarters in Foster City, California.

Contacts
Jacquie Ross, Investors
(408) 656-8793
Mark Snyder, Media
(650) 450-2480
|
|
| |
| |
|
|
|