| |
CDC TPOXX Study Results Monkeypox Treatment
|
| |
| |
CDC MMWR
Clinical Use of Tecovirimat (Tpoxx) for Treatment of Monkeypox Under an Investigational New Drug Protocol - United States, May-August 2022
Early Release / September 9, 2022
https://www.cdc.gov/mmwr/volumes/71/wr/mm7137e1.htm
• For patients treated under the EA-IND protocol and included in this report, the median time to subjective improvement was 3 days after receiving tecovirimat. However, no control group was available for comparison; therefore, no conclusions can be drawn regarding the effectiveness of tecovirimat to treat monkeypox based on these data.
• Interim CDC guidance currently recommends that tecovirimat be considered in patients with severe disease, those at high risk for severe disease, or those with aberrant infections. This report describes the available demographic and clinical characteristics, clinical indications for use, clinical outcomes, and adverse events reported among some of the first known recipients of tecovirimat treatment under the EA-IND protocol for Monkeypox virus infection in the United States.
• Ongoing monitoring is essential to assess the safety of tecovirimat in patients with Monkeypox virus infection under the EA-IND during the current monkeypox outbreak. CDC is continuing to review additional data as they become available. Currently, there are no human data demonstrating the efficacy of tecovirimat, and clinical trials are necessary to elucidate clinical efficacy in patients with Monkeypox virus infection, indications for treatment, and ideal duration of treatment.
• Approximately one half of patients with Monkeypox virus infection who received tecovirimat were living with HIV infection.
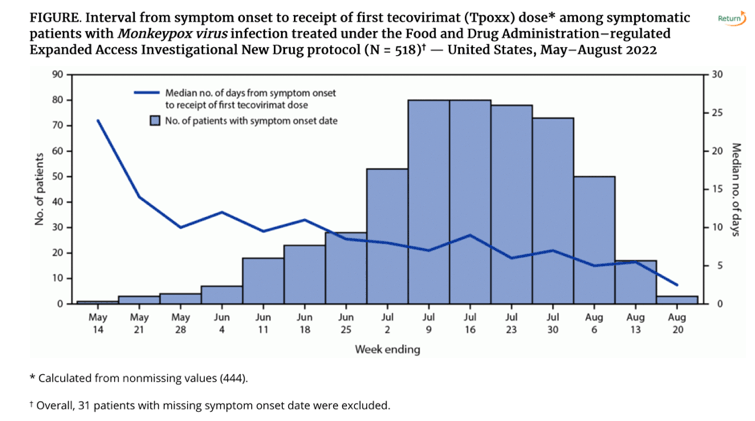
• the oral formulation of tecovirimat was prescribed to 494 (99.8%) at the start of therapy. Median interval from symptom onset to receipt of first tecovirimat dose was 7 days (IQR = 5-10 days) (Figure).
• CDC abstracted data from patient intake forms for 549 persons with confirmed or suspected monkeypox who were prescribed tecovirimat therapy by August 20, 2022, and outcome forms for 369 patients.
• Among 464 patients with race and ethnicity data, 180 (38.8%) were White persons, 161 (34.7%) were Hispanic or Latino persons, and 83 (17.9%) were non-Hispanic Black or African American persons.
• Among 317 patients with available outcome information, 230 (72.6%) recovered with or without sequelae
• For patients treated under the EA-IND protocol and included in this report, the median time to subjective improvement was 3 days after receiving tecovirimat.
• Among 529 patients with available data on number of lesions, 299 (56.5%) reported 10-100 lesions at the start of tecovirimat; 210 (39.7%) had fewer than 10 lesions, and 20 (3.8%) had more than 100 lesions. The presence of lesions in anatomic areas that might result in serious sequelae was reported on 191 (79.6%) of the 240 revised intake forms with available data. The most frequently reported underlying medical condition affecting immune status was HIV infection (254, 46.3%); viral load and CD4 count were not reported.
• Among 495 persons with available data on route of administration, the oral formulation of tecovirimat was prescribed to 494 (99.8%) at the start of therapy. Median interval from symptom onset to receipt of first tecovirimat dose was 7 days (IQR = 5-10 days) (Figure). Among 260 persons with revised intake forms, 124 (47.7%) had laboratory-confirmed Orthopoxvirus infection when tecovirimat treatment commenced.
• Among 369 patients with outcome forms, data on hospitalization status was available for 331; among these, 23 (6.9%) were hospitalized after symptom onset (Table 2), and the median duration of hospitalization was 4 days (IQR = 1-5 days). Among 255 patients with available data, the median time to subjective improvement after starting treatment was 3 days (IQR = 2-4 days). Among 317 patients with available outcome information, 230 (72.6%) recovered with or without sequelae**** by or before completion of the posttreatment assessment; 87 (27.4%) patients were reported by clinicians to be not yet recovered, 78 of whom had not yet completed the standard 14-day tecovirimat treatment course. Adverse events were reported for 12 (3.5%) of 340 patients with information on adverse events; these included headache (three), nausea (two), visual disturbance (two), weakness (two), and hospitalization for psychiatric reasons (one).
• At the time of the posttreatment follow-up visit, three (2.2%) of 137 persons with information available had developed new lesions compared with 25 (13.1%) who had developed new lesions during the first week of treatment. Most (119, 89.5%) patients reported that all lesions were crusted and healing with a new layer of skin under the scab following treatment. Among 174 patients with available data, the interval to subjective improvement did not differ between HIV-positive persons (42; median = 3 days) and persons without documentation of HIV positive status (64; median = 3 days) with available data (p = 0.83).
---------------------------
Summary
What is already known about this topic?
Tecovirimat (Tpoxx) was approved by the Food and Drug Administration for treatment of smallpox based on data obtained from animal models; there are no safety or efficacy data regarding its use in patients with Monkeypox virus infection.
What is added by this report?
Among 549 patients with Monkeypox virus infection treated with tecovirimat under an Expanded Access Investigational New Drug protocol, 99.8% received it orally as an outpatient. Among 369 patients, few adverse events were reported.
What are the implications for public health practice?
Tecovirimat is generally well tolerated, and these data support continued access to treatment with tecovirimat during the current monkeypox outbreak.
Currently, no Food and Drug Administration (FDA)-approved treatments for human monkeypox are available. Tecovirimat (Tpoxx), however, is an antiviral drug that has demonstrated efficacy in animal studies and is FDA-approved for treating smallpox. Use of tecovirimat for treatment of monkeypox in the United States is permitted only through an FDA-regulated Expanded Access Investigational New Drug (EA-IND) mechanism. CDC holds a nonresearch EA-IND protocol that facilitates access to and use of tecovirimat for treatment of monkeypox.§ The protocol includes patient treatment and adverse event reporting forms to monitor safety and ensure intended clinical use in accordance with FDA EA-IND requirements. The current multinational monkeypox outbreak, first detected in a country where Monkeypox virus infection is not endemic in May 2022, has predominantly affected gay, bisexual, and other men who have sex with men (MSM) (1,2). To describe characteristics of persons treated with tecovirimat for Monkeypox virus infection, demographic and clinical data abstracted from available tecovirimat EA-IND treatment forms were analyzed. As of August 20, 2022, intake and outcome forms were available for 549 and 369 patients, respectively; 97.7% of patients were men, with a median age of 36.5 years. Among patients with available data, 38.8% were reported to be non-Hispanic White (White) persons, 99.8% were prescribed oral tecovirimat, and 93.1% were not hospitalized.
Approximately one half of patients with Monkeypox virus infection who received tecovirimat were living with HIV infection. The median interval from initiation of tecovirimat to subjective improvement was 3 days and did not differ by HIV infection status. Adverse events were reported in 3.5% of patients; all but one adverse event were nonserious. These data support the continued access to and treatment with tecovirimat for patients with or at risk for severe disease in the ongoing monkeypox outbreak.
During May 29-July 20, 2022,
|
|
| |
| |
|
|
|