 |
 |
 |
| |
ViiV HEALTHCARE TO PRESENT NEW DATA FROM INNOVATIVE HIV TREATMENT AND PREVENTION PORTFOLIO AT AIDS 2022
|
| |
| |
Data to be presented include long-term and real-world data from portfolio of medicines, including long-acting and 2-drug regimens
London, 25 July 2022 - ViiV Healthcare, the global specialist HIV company majority-owned by GSK, with Pfizer and Shionogi as shareholders, today announced the presentation of 29 abstracts from the company's diverse portfolio of licensed treatment and prevention options at the 24th International AIDS Conference (AIDS 2022) being held in Montreal, Canada from 29 July - 2 August.
Kimberly Smith, M.D., MPH, Head of Research & Development at ViiV Healthcare, said: "The needs of people living with HIV continue to evolve, and the broad range of data to be presented at AIDS 2022 illustrates ViiV Healthcare's leadership and commitment to innovation in addressing these changing needs. We're excited to be presenting new data related to HIV prevention, real-world data from long-acting and 2-drug regimens, studies that are specifically aimed at understanding the impact of antiretrovirals among older patients and long-term findings for heavily treatment-experienced patients. We look forward to connecting in person at this year's meeting and sharing these data with the scientific and HIV communities."
Key abstracts to be presented at AIDS 2022 by ViiV Healthcare and its study partners will include:
• Updated efficacy and safety results from the HPTN 084 study of cabotegravir long-acting for PrEP in cisgender women1:Findings to be presented at AIDS 2022 will report on outcomes from the 12-month period following trial unblinding, including the rate of reduction in HIV incidence of cabotegravir, an updated efficacy reading compared to emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) tablets, and pregnancy outcomes.
• An analysis of efficacy, safety, and gender affirming hormonal therapy (GAHT) interactions among transgender women in the HPTN 083 study of long-acting cabotegravir for PrEP2: Findings to be presented at AIDS 2022 will include an evaluation of the impact of GAHT on cabotegravir in a subset of transgender women with and without GAHT. The analysis also includes a report on a comparison of participant characteristics, including history of interpersonal violence, HIV risk perception, and grade 2+ adverse events, between transgender women and the larger HPTN 083 study population.
• Week 240 results of the phase III BRIGHTE study investigating the efficacy and safety of fostemsavir in heavily treatment-experienced adults with HIV3, which build upon earlier 96-week efficacy and safety data. Long-term results to be presented will share virologic response rates through week 240 in multi-drug resistant adults living with HIV, with mean CD4+ cell counts along with adverse events.
• Real-world outcomes from the CARLOS study evaluating switching to long-acting cabotegravir and rilpivirine for the treatment of HIV4: Findings to be presented include an analysis of reasons for switching to long-acting therapy from the perspectives of people living with HIV and healthcare providers, and feature the most common reason for initiating cabotegravir and rilpivirine among these groups.
• Safety and effectiveness outcomes from the CARISEL study on cabotegravir and rilpivirine long-acting integration into European clinical settings5: Findings to be presented will demonstrate that cabotegravir and rilpivirine long-acting administered every two months was well tolerated and highly effective in maintaining virologic suppression, with a low rate of virologic failure, across diverse European clinical settings and participants.
• Real-world outcomes from the TANDEM study evaluating dolutegravir-based 2-drug regimens for the treatment of HIV6:Findings to be presented feature the most common reason for initiating dolutegravir/lamivudine (DTG/3TC) and an analysis of real-world results among treatment-naïve people living with HIV initiating a DTG/3TC regimen and people living with HIV switching to a DTG/3TC regimen.
• 48-week results from the SALSA study analysing patient-reported outcomes in older adults after switching to a 2-drug regimen of dolutegravir/lamivudine (DTG/3TC)7: Findings to be presented include patient-reported outcomes in virologically suppressed adults over the age of 50 living with HIV on a current antiretroviral regimen (CAR) consisting of at least three drugs on the DTG/3TC regimen including symptom distress module scores, HIV treatment satisfaction, and lifestyle/ease sub-score.
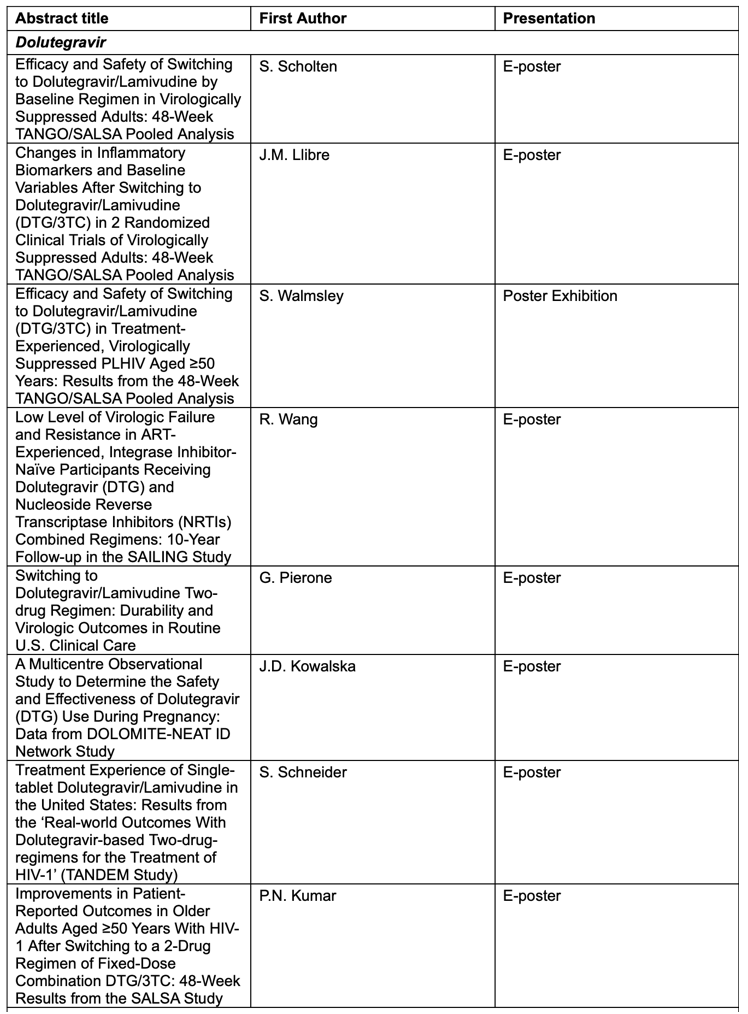
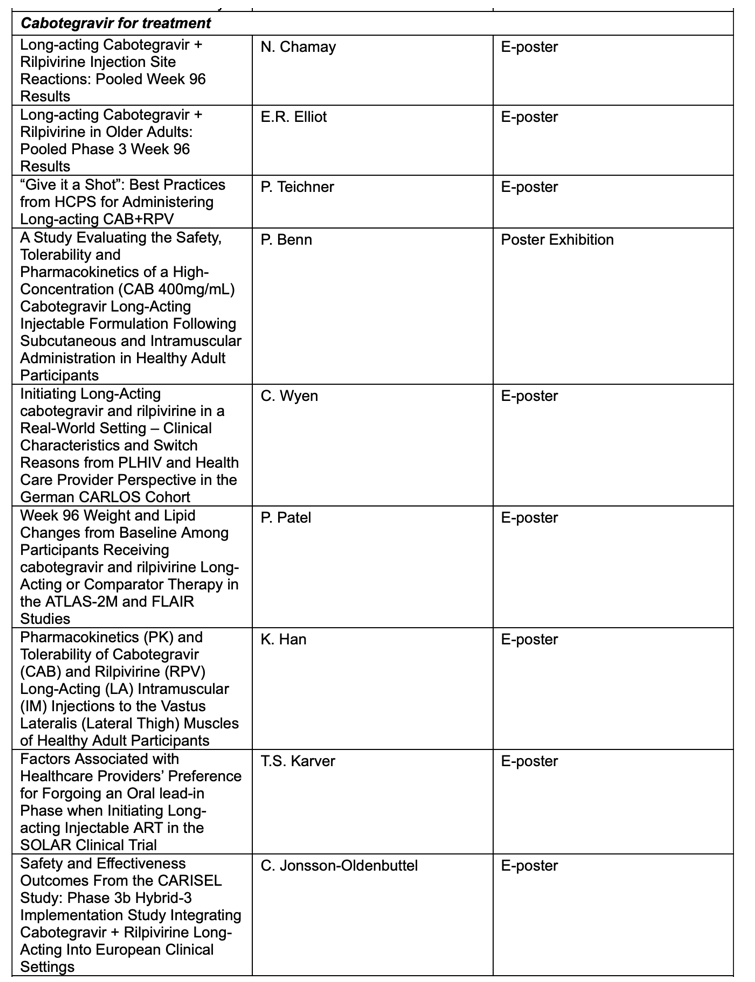
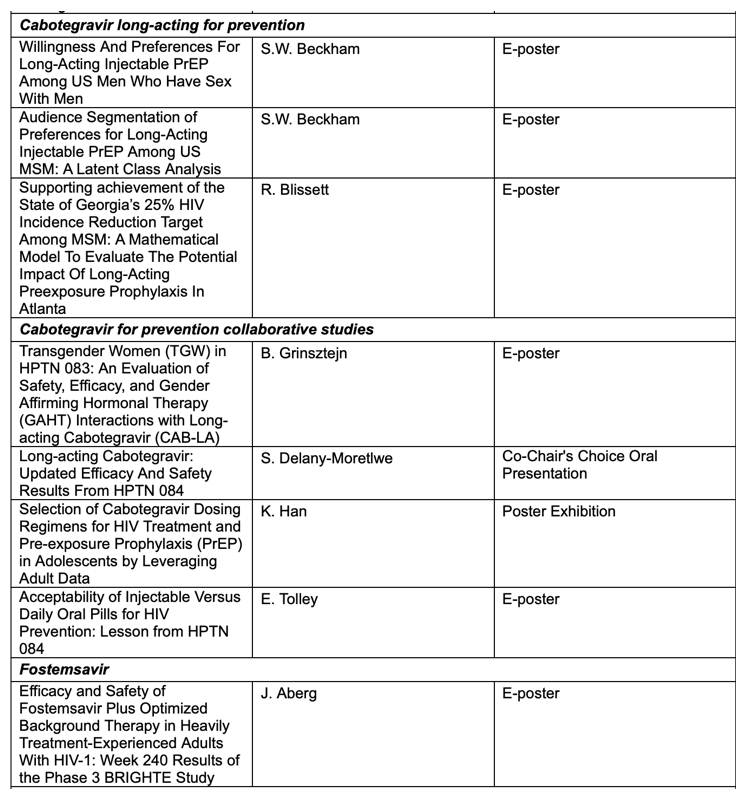
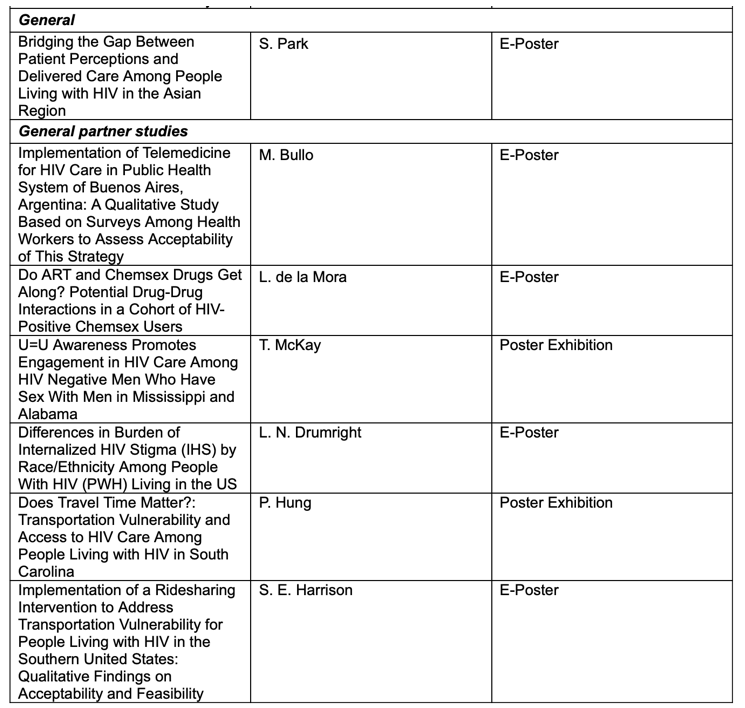
Here is a list of ViiV Healthcare sponsored and supported studies to be presented at AIDS 2022:
Important Safety Information for Dovato (50mg dolutegravir/300mg lamivudine) Tablets
INDICATION
Dovato is indicated as a complete regimen to treat HIV-1 infection in adults with no antiretroviral (ARV) treatment history or to replace the current ARV regimen in those who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable ARV regimen with no history of treatment failure and no known resistance to any component of Dovato.
IMPORTANT SAFETY INFORMATION
BOXED WARNING: PATIENTS CO-INFECTED WITH HEPATITIS B VIRUS (HBV) AND HIV-1: EMERGENCE OF LAMIVUDINE-RESISTANT HBV AND EXACERBATIONS OF HBV
All patients with HIV-1 should be tested for the presence of HBV prior to or when initiating Dovato. Emergence of lamivudine-resistant HBV variants associated with lamivudine-containing antiretroviral regimens has been reported. If Dovato is used in patients co-infected with HIV-1 and HBV, additional treatment should be considered for appropriate treatment of chronic HBV; otherwise, consider an alternative regimen.
Severe acute exacerbations of HBV have been reported in patients who are co-infected with HIV-1 and HBV and have discontinued lamivudine, a component of Dovato. Closely monitor hepatic function in these patients and, if appropriate, initiate anti-HBV treatment.
Contraindications
• Do not use Dovato in patients with previous hypersensitivity reaction to dolutegravir or lamivudine
• Do not use Dovato in patients receiving dofetilide
See full press announcement:
https://viivhealthcare.com/hiv-news-and-media/news/press-releases/2022/july/viiv-healthcare-to-present-new-data-from-innovative-hiv-treatment/
|
| |
|
 |
 |
|
|