 |
 |
 |
| |
Association of prenatal PrEP exposure with neurodevelopmental and growth outcomes beyond 24 months among Kenyan children
|
| |
| |
AIDS 2022 July 29-Aug 2 Montreal

Presenter
Lauren Gomez
Authors
L. Gómez * (1), J. Kinuthia (2), A. Larsen (1), J. Stern (1), J. Dettinger (1), M. Marwa (2), F. Abuna (2), B. Ochieng (2), N. Ngumbau (2), P. Omondi (2), G. John-Stewart (1), J. Pintye (1)
Institutions
(1) University of Washington, Seattle, United States, (2) Kenyatta National Hospital, Nairobi, Kenya
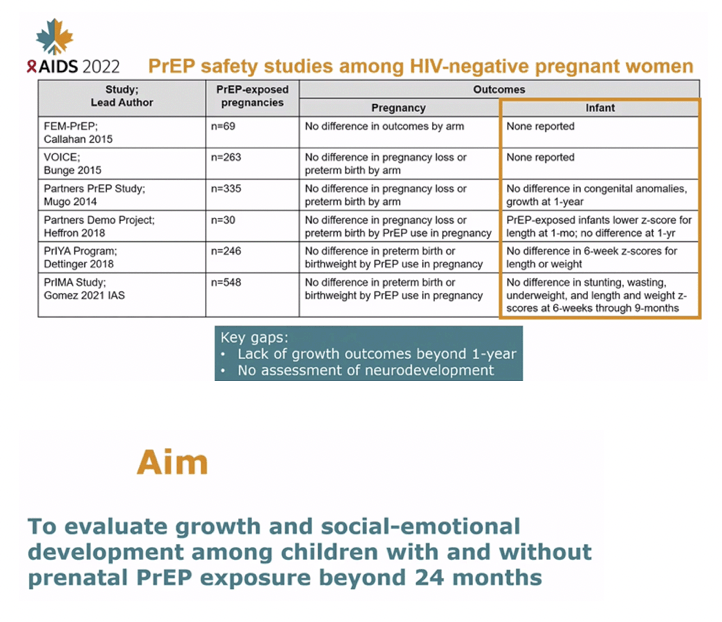
BACKGROUND: Safety data of prenatal PrEP use are reassuring, yet studies to date have less than 1 year of follow-up and do not assess neurodevelopmental outcomes among PrEP-exposed infants. Evaluating safety outcomes beyond infancy following maternal PrEP use could help complete the safety profile for PrEP use during pregnancy.
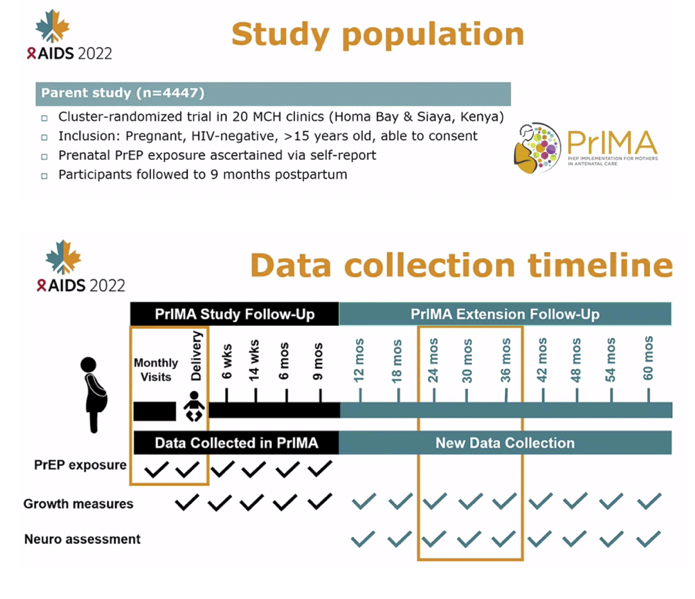
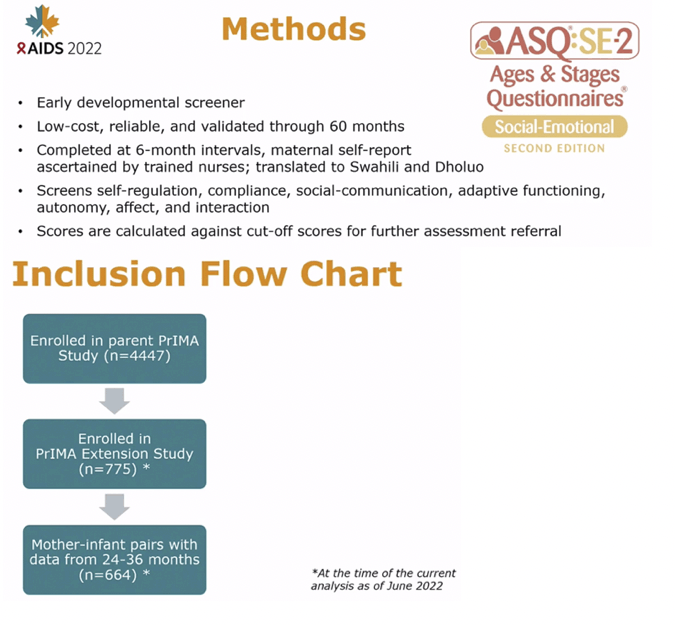
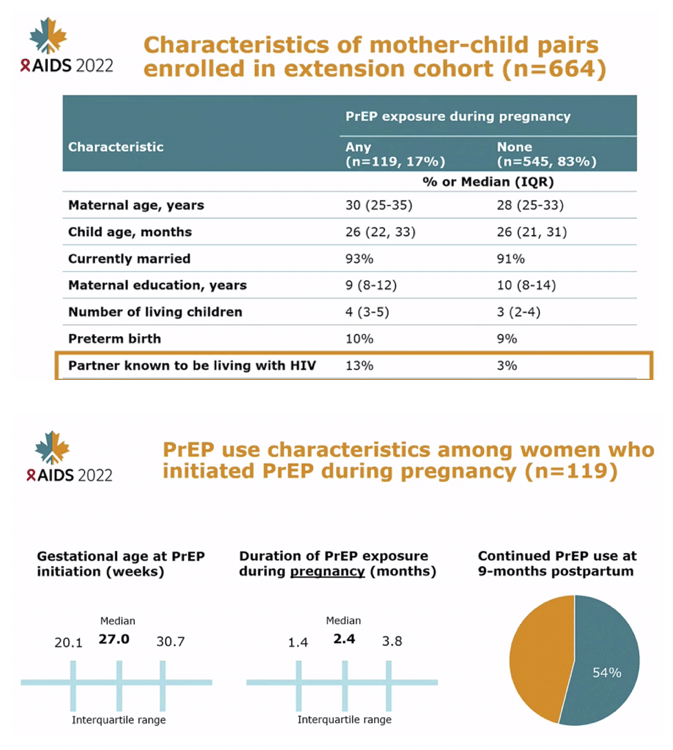
METHODS: We utilized data from mother-child pairs enrolled in an ongoing evaluation of perinatal PrEP use in Western Kenya. In the parent study (NCT03070600), HIV-negative women were enrolled and offered PrEP during pregnancy at 20 public sector maternal child health (MCH) clinics and followed through 9 months postpartum regardless of PrEP status. An extension cohort to evaluate safety outcomes enrolled mother-child pairs at 4 sites to be followed until the child's 5th birthday. Between October 2020 and January 2022, trained study nurses conducted anthropometric measurements on children and assessed neurodevelopment using the Ages and Stages Questionnaire (ASQ), an early developmental screener. Using data from 24-30 months, we evaluated the association of prenatal PrEP exposure and growth and ASQ scores using linear regression models, clustered by facility, and adjusted for gestational age at birth.
RESULTS: Among 472 mother-child pairs included in the analysis, median maternal age was 27.8 years (IQR: 24.6-33.0) and median child age was 25 months (IQR: 21-28) at enrollment into the extension cohort; 16.3% had any PrEP exposure during pregnancy for a median duration of 3.0 months (IQR: 2.0-4.3).
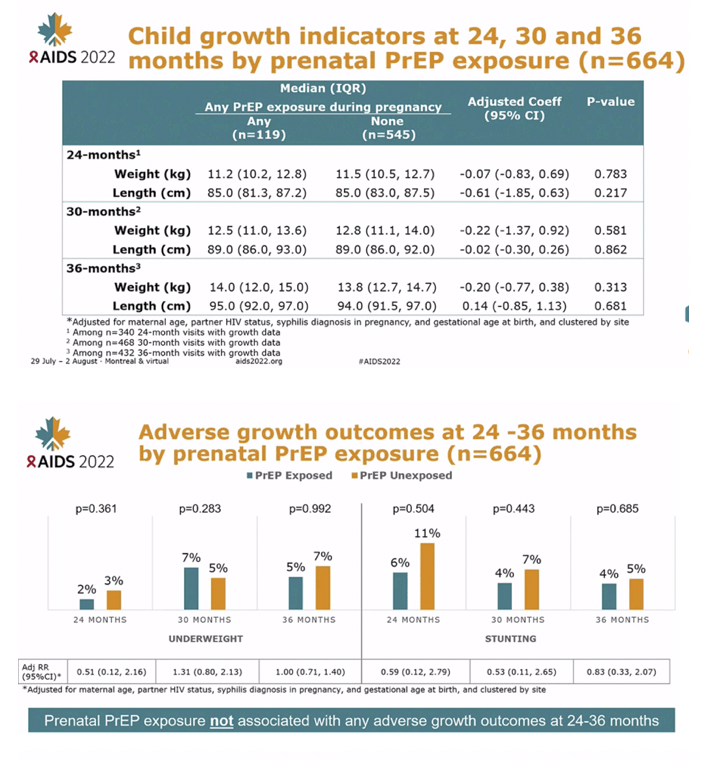
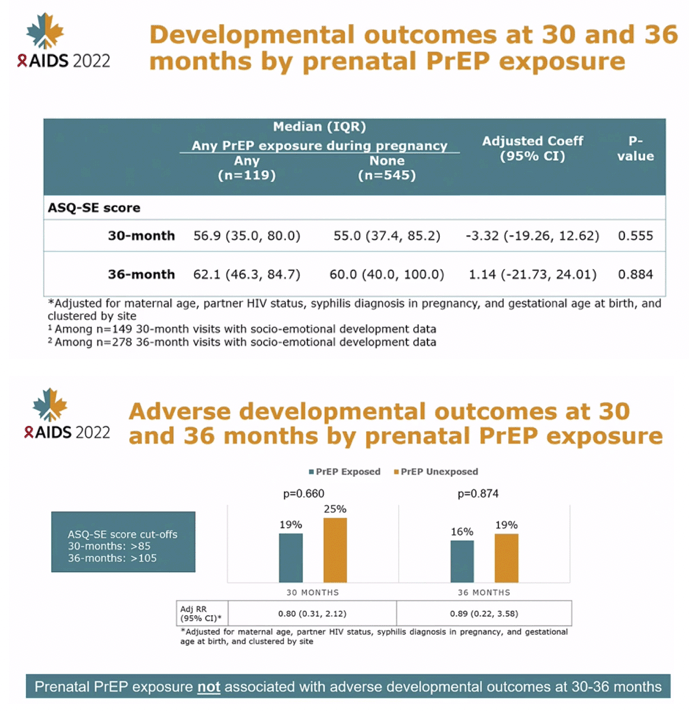
At 24-month visits, there was no difference in mean weight (mean difference -0.04 kg, 95% CI: -0.78, 0.70, p=0.886), mean height (mean difference -0.43 cm, 95% CI: -2.32, 1.46, p=0.520), frequency of underweight (5.6% vs. 4.0%, adjusted prevalence ratio[aPR]=1.49, 95% CI: 0.27-8.09, p=0.647) and frequency of stunting (22.2% vs. 21.4%, aPR=1.07, 95%CI: 0.64-1.78, p=0.805) between children with and without prenatal PrEP exposure. Results were similar at 30-month visits. Prenatal PrEP exposure was not associated with overall ASQ scores at 24-months (p=0.243) or 30-months (p=0.664).
CONCLUSIONS: Among Kenyan mother-child pairs followed from pregnancy through early childhood, we found no differences in growth or neurodevelopmental outcomes between children with and without prenatal PrEP exposure. Our results support prior data indicating safety of prenatal PrEP use.

|
| |
|
 |
 |
|
|