 |
 |
 |
| |
B/F/TAF an Effective and Safe
Switch for HIV/HBV: 24-Week Results
|
| |
| |
IDWeek 2022, October 19-23, 2022, Washington, DC
Mark Mascolini
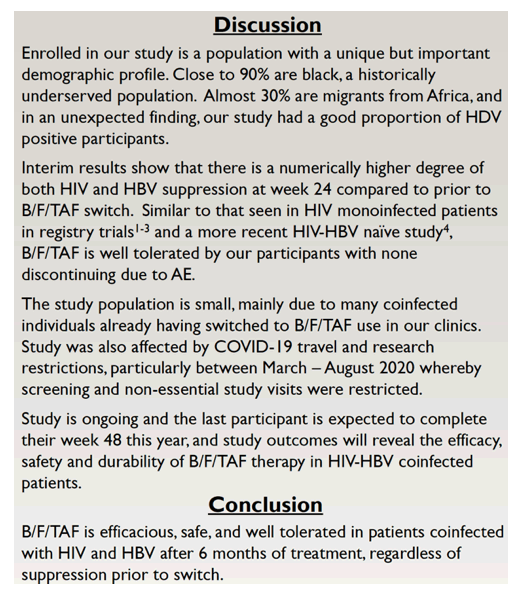
Suppression of HIV and HBV improved over 24 weeks in people switching to bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in a small single-arm study of coinfected people at two clinics in Baltimore and Philadelphia [1]. Viral suppression rates were similar to those in a study of treatment-naive people with HIV and HBV [2].
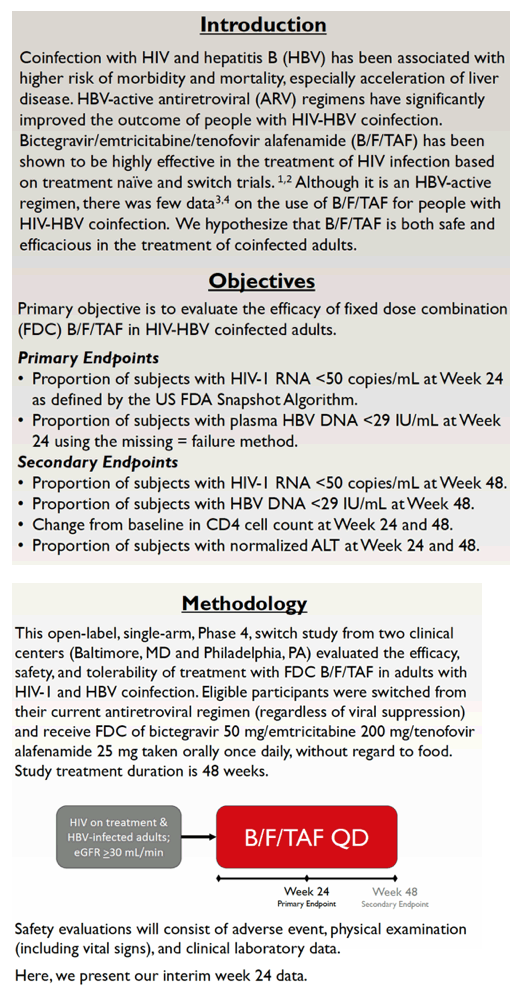
Although B/F/TAF includes two components-emtricitabine and tenofovir alafenamide-active against HBV, research on its efficacy and safety in people coinfected with HIV and HBV remains limited [2,3]. Collaborators in the Philadelphia Department of Public Health and the University of Maryland are conducting this open-label, single-arm switch study at two clinics in Baltimore and Philadelphia.
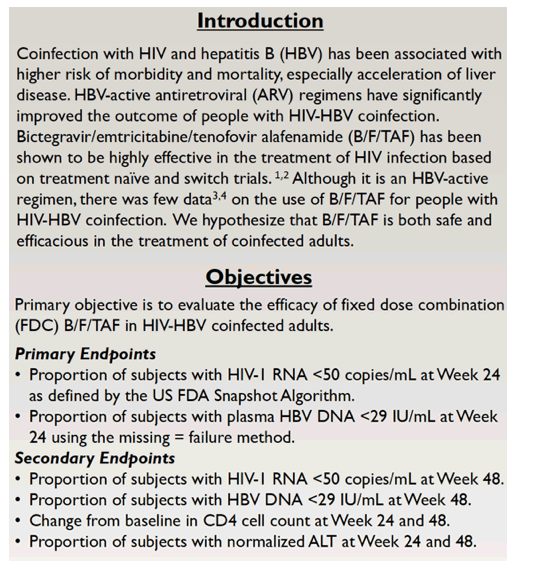
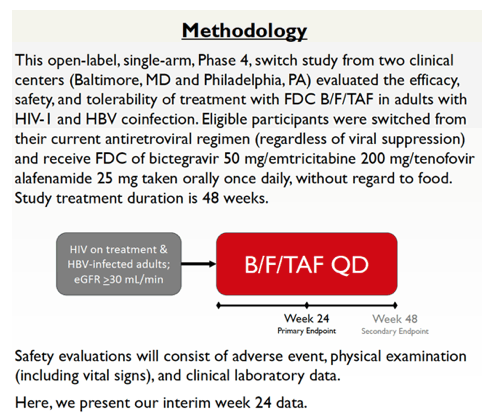
The study enrolled people being treated for HIV infection and coinfected with HBV. Everyone had an estimated glomerular filtration rate at or above 30 mL/min, and everyone switched to B/F/TAF once daily regardless of suppression on their current combination. The primary endpoints were the proportion of participants with (1) an HIV load below 50 copies at treatment week 24 according to FDA snapshot analysis and (2) an HBV load below 29 IU/mL in a missing-data-equals-failure analysis.
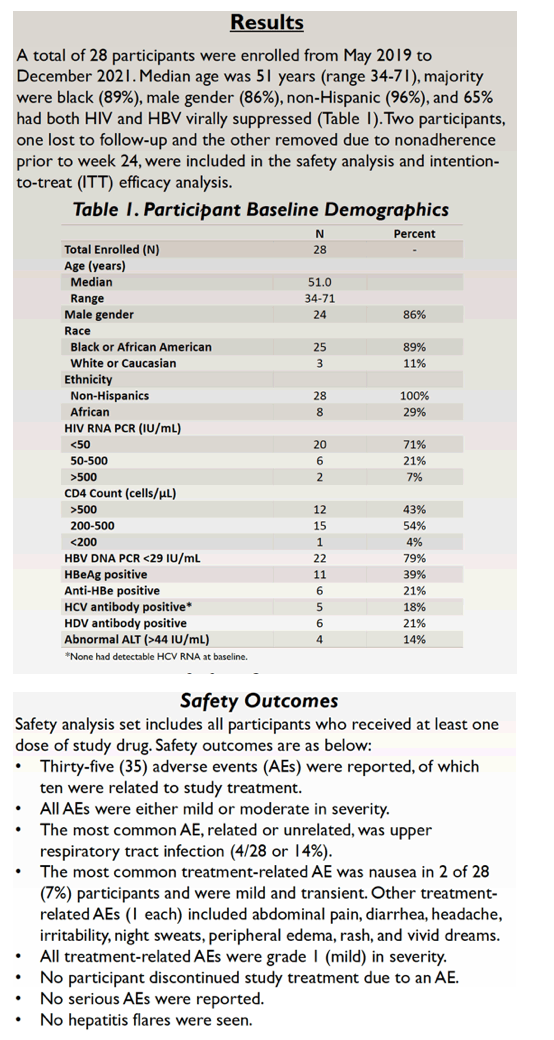
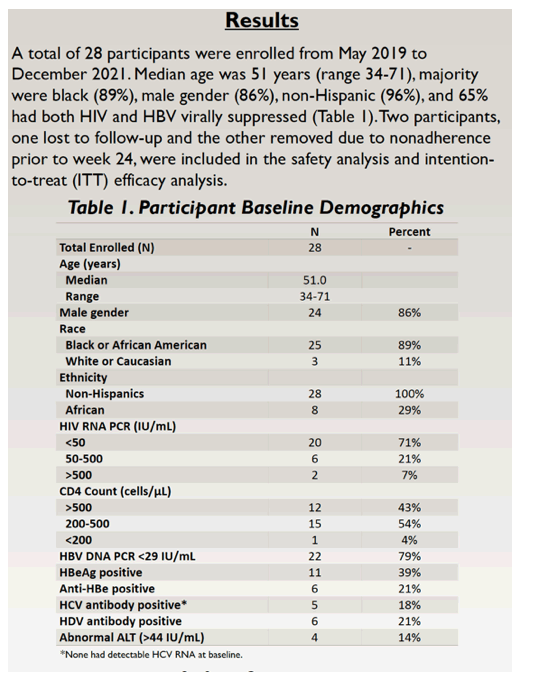
Researchers enrolled 28 people from May 2019 to December 2021. The group had a median age of 51 years (range 34 to 71), 86% were men, 89% black, and 11% white. Twenty participants (71%) had an HIV load below 50 copies at the switch, 6 (21%) had a load between 50 and 500 copies, and the remaining 2 had a load above 500 copies. Twenty-two people (79%) had an HBV load below 29 IU/mL, 11 (39%) were HBeAg positive, 6 (21%) were anti-HBe positive, 5 (18%) were HCV antibody positive, and 6 (21%) were HDV antibody positive. Only 4 people (14%) had an abnormal alanine aminotransferase (ALT, above 44 IU/mL).
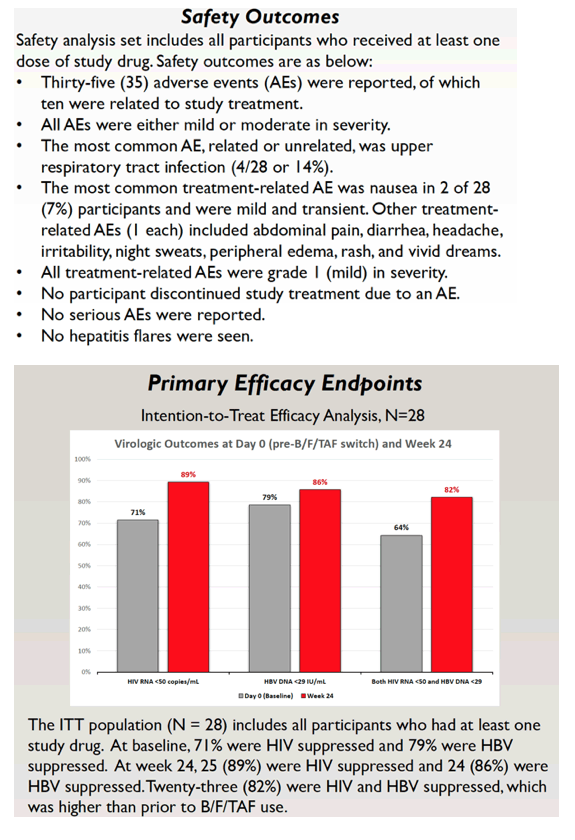
Safety results came from people who got at least one dose of B/F/TAF. Of the 35 adverse events, researchers judged 10 of them related to study drugs, and all were mild or moderate. The most frequent treatment-related problem was mild and transient nausea in 2 of 28 people. All treatment-related adverse events were grade 1 (mild). No one stopped treatment because of an adverse event, and no one had a hepatitis flare. The 4 elevated ALTs observed at the switch to B/F/TAF returned to normal at week 24.
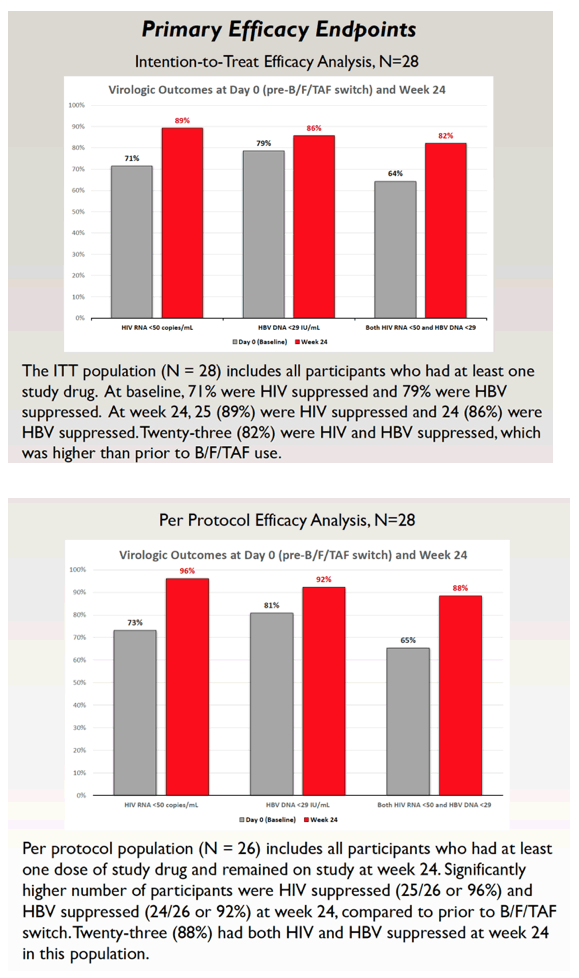
Among the 28 people in the intention-to-treat population (those who took at least once dose of B/F/TAF), 71% had HIV suppression and 79% had HBV suppression at the switch to B/F/TAF. At treatment week 24, 25 people (89%) attained HIV suppression and 24 (86%) had HBV suppression. Twenty-three participants (82%) had both HIV and HBV suppression, compared with 18 (64%) before the switch.
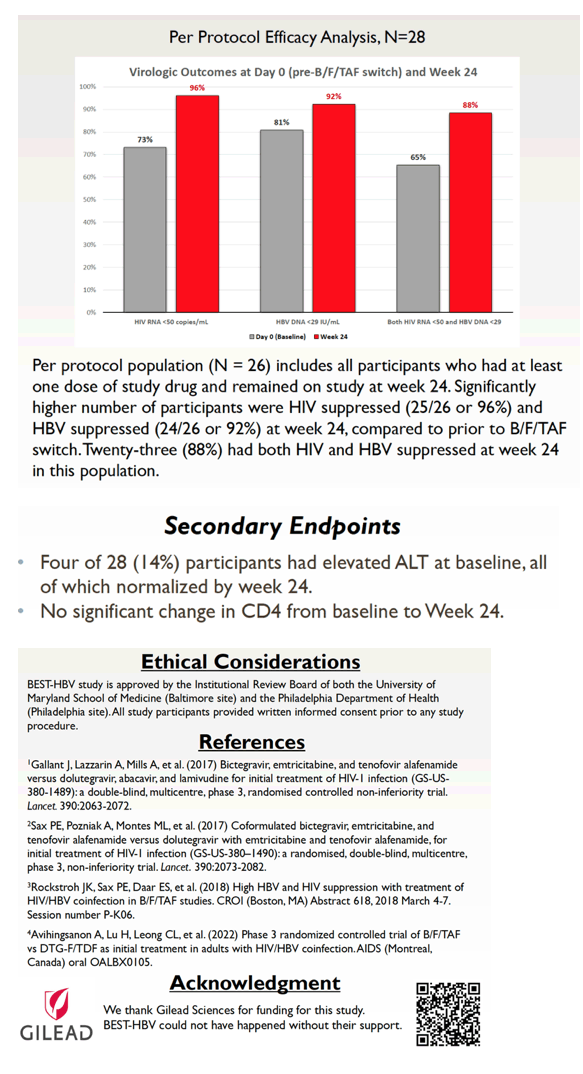
A per-protocol analysis involved 26 people who took at least one dose and remained in the study at week 24. At that point, 25 of 26 (96%) had HIV suppression and 24 of 26 (92%) had controlled HBV. Those percentages compared with baseline figures of 73% and 81%. At the 24-week point, 23 of 26 people (88%) attained suppression of both HIV and HBV, compared with 17 of 26 (65%) at the switch.
The researchers expect all participants to reach 48 weeks of follow-up during 2022. On the basis of these preliminary results, they proposed that "B/F/TAF is efficacious, safe, and well tolerated" in HIV/HBV-coinfected people regardless of viral load when switching to this triple combination.
References
1. Kwakwa H, Bran J, Ruff J, Choe S, Chua JV. Efficacy, safety, and tolerability of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in adults with HIV-HBV infection-preliminary results. IDWeek 2022, October 19-23, 2022, Washington, DC. Abstract 1268.
2. Avihingsanon A, Lu H, Leong CL, et al. Phase 3 randomized controlled trial of B/F/TAF vs DTG-F/TDF as initial treatment in adults with HIV/HBV coinfection. AIDS 2022. Montreal. Abstract OALBX0105. https://www.natap.org/2022/IAC/IAC_29.htm
3. Rockstroh JK, Sax PE, Daar ES, et al. High HBV and HIV suppression with treatment of HIV/HBV coinfection in B/F/TAF studies. CROI, March 4-7, 2018, Boston. Abstract 618. https://www.natap.org/2018/CROI/croi_73.htm
|
| |
|
 |
 |
|
|