 |
 |
 |
| |
Switching to an Integrase Inhibitor Tied to Liver Fibrosis Score in US Women
|
| |
| |
Mark Mascolini
IDWeek 2022, October 19-23, 2022, Washington, DC
A study of 872 US women with HIV linked starting an integrase inhibitor to worse NAFLD Fibrosis Score (NFS) and to rising fibrosis risk reflected in NFS and another noninvasive measure, Fibrosis-4 (FIB-4) [1]. But a third noninvasive fibrosis test, AST-to-platelet ratio (APRI), indicated lower chances of fibrosis 3 to 4 years after switching to an integrase inhibitor.
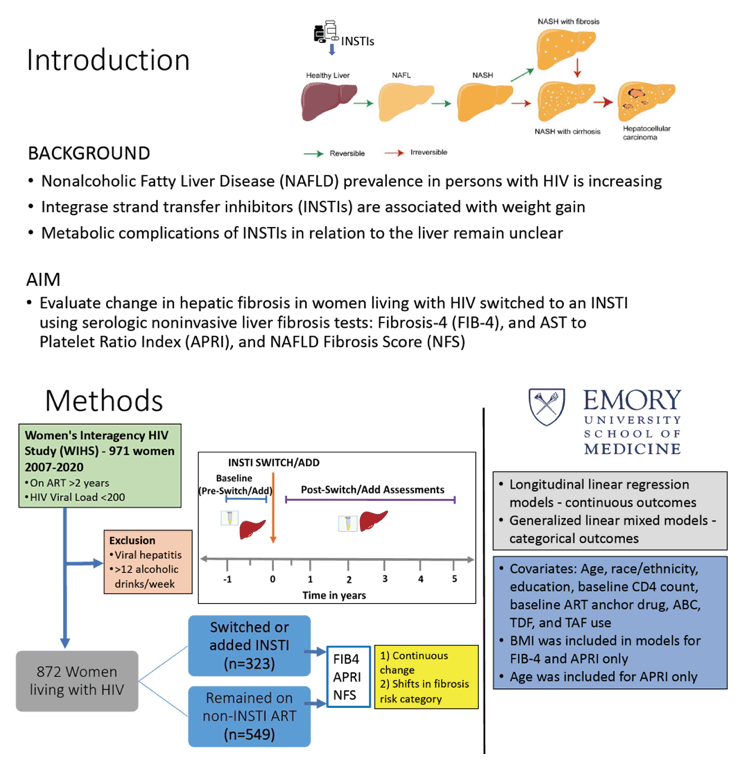
Researchers working with the Women's Interagency HIV Study (WIHS) reminded colleagues that prevalence of nonalcoholic fatty liver disease (NAFLD) stands above 30% in people with HIV, and that rate is on the way up. NAFLD risk factors include antiretrovirals, notably integrase inhibitors, which research ties to weight gain and metabolic meanderings. They conducted this analysis of WIHS women to track changes in liver fibrosis among women switching to an integrase inhibitor, using the noninvasive tests FIB-4, APRI, and NFS.
The analysis compared 323 WIHS women with HIV who switched to an integrase inhibitor and 549 women who stayed with their no-integrase combination. The investigators excluded women with untreated viral hepatitis, heavy alcohol use, or other causes of chronic liver disease. They recorded findings with the three fibrosis tests before and after women switched to an integrase inhibitor. The WIHS team used longitudinal linear regression to analyze continuous outcomes and generalized linear mixed models to analyze categorical outcomes. These statistical models adjusted for age, body mass index, race/ethnicity, education, baseline CD4 count, baseline antiretroviral anchor drug (protease inhibitor or nonnucleoside), baseline antiretroviral backbone (TDF, TAF, or abacavir), and alcohol use.
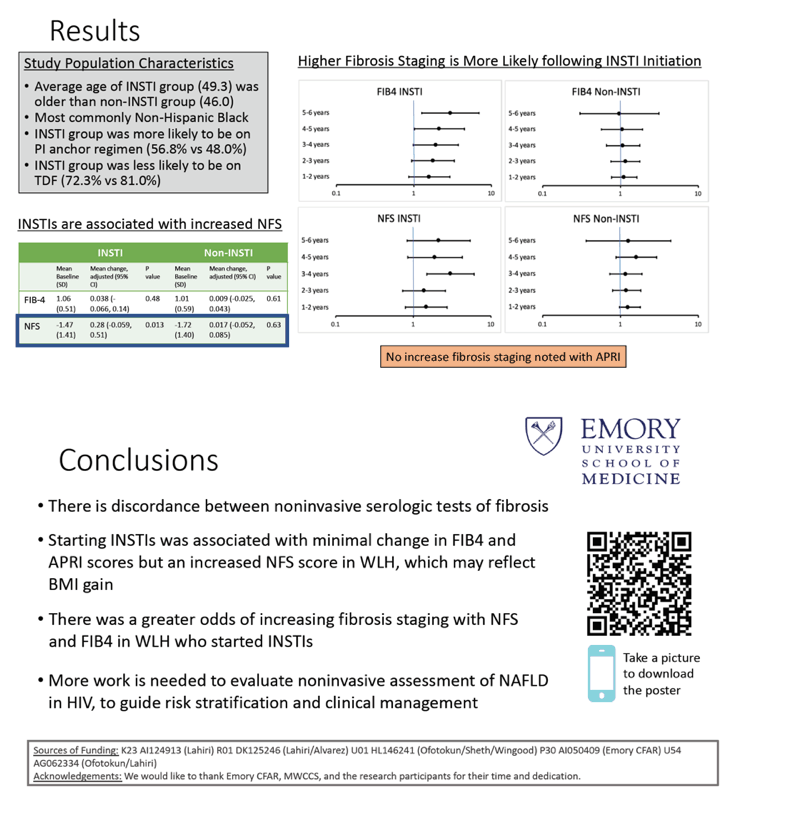
Compared with women who did not switch to an integrase inhibitor, those who did were older (average 49.3 vs 46.0, P < 0.001), more likely to be white (66.9% vs 58.5%), less likely to be Hispanic (17.0% vs 26.5%), and about equally likely to be black (16.1% and 15.0%) (P = 0.056). While 28% of each group smoked cigarettes, about two thirds abstained from alcohol and only about 2% in each group drank heavily. About 87% of each group had an undetectable HIV load, and about 90% of each group had better than 95% antiretroviral adherence.
In women switching to an integrase inhibitor, average adjusted score did not change significantly over up to 5 or 6 years after the switch with FIB-4 or APRI but increased significantly with NFS, from -1.47 to +0.28 (P = 0.013). Over a similar period, average adjusted fibrosis score changed little with any of the three methods in women who stayed on the same antiretrovirals.
The multivariate model figured greater odds of worsening fibrosis in integrase inhibitor switchers with either FIB-4 or NFS, particularly as time after the switch lengthened. But the same model determined that the APRI score indicated lower odds of fibrosis starting 3 to 4 years after women switched to an integrase inhibitor.
The WIHS investigators suggested the rising odds of fibrosis seen with FIB-4 or NFS could reflect gains in body mass index among these women after they swapped an antiretroviral in their regimen for an integrase inhibitor. They proposed that noninvasive assessment of NAFLD in people with HIV should ideally be correlated with liver biopsy results, the gold standard of liver measures. They also suggested that vibration-controlled transient elastography could assess steatosis and fibrosis changes after starting an integrase inhibitor.
Reference
1. Yu MA, Gerig L, Mehta CC, et al. Noninvasive assessment of change in hepatic fibrosis following initiation of integrase inhibitors in women living with HIV. IDWeek 2022, October 19-23, 2022, Washington, DC. Abstract 1276.
|
| |
|
 |
 |
|
|