 |
 |
 |
| |
Tenofovir versus entecavir in preventing HCC and death:
answer from a large integrated health plan population
|
| |
| |
Matched Analysis Shows Anti-HBV TDF No Better Than ETV in HCC or Death Outcomes
AASLD 2023, The Liver Meeting, November 10-14, 2023, Boston
https://www.aasld.org/the-liver-meeting
Mark Mascolini
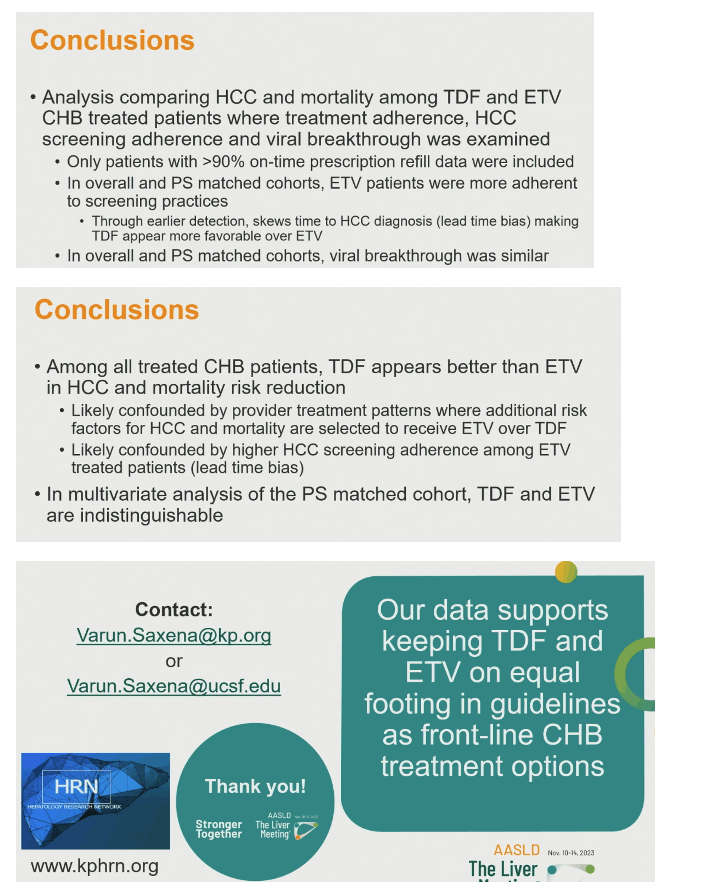
In a large cohort treated with tenofovir disoproxil (TDF) or entecavir (ETV) for chronic HBV infection, propensity score matching indicated no difference between the drugs in new diagnoses of hepatocellular carcinoma (HCC) or in all-cause mortality [1]. This analysis from the Kaiser Permanente Northern California healthcare system found an advantage for TDF in an unmatched comparison, but propensity score matching suggested that more risk factors and better HCC screening adherence in people prescribed ETV explained the difference.
Varun Saxena and Kaiser colleagues noted that guidelines recommend either TDF or ETV for first-line therapy of chronic HBV infection. Some studies found TDF superior to ETV in preventing HCC but others did not [2]. The Kaiser investigators suggested that these studies were limited by incomplete statistical adjustment for confounders and lack of data on ETV or TDF adherence or HCC screening adherence.
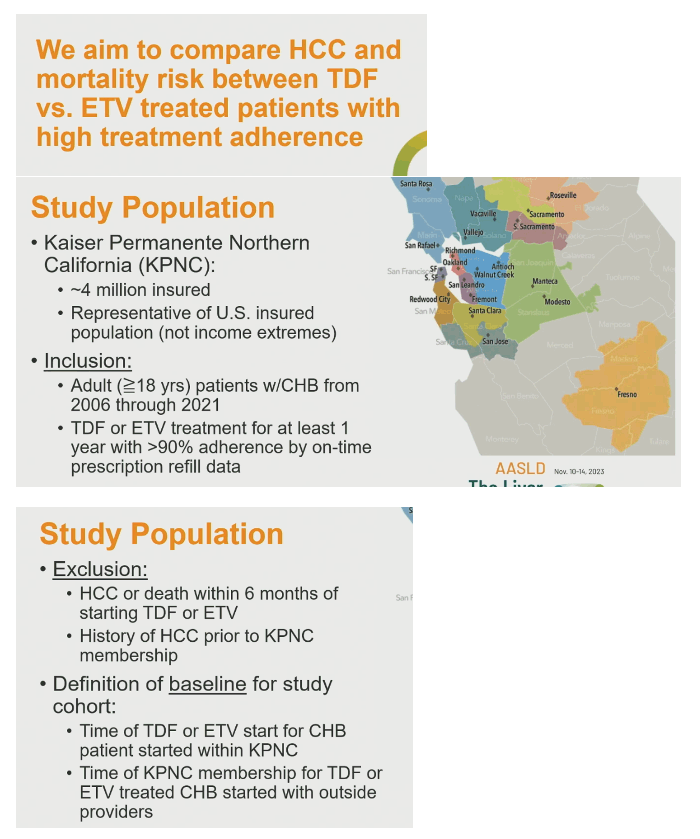
To shed more light on these questions, the Saxena and coworkers analyzed records of Kaiservhealthcare system members at least 18 years old and treated with TDF or ETV for chronic HBV infection for at least 1 year from 2006 through 2021. Prescription refill data indicated better than 90% adherence for each study participant. The researchers excluded people who got an HCC diagnosis or died within 6 months of starting TDF or ETV and people with a history of HCC before entering the Kaiser system.
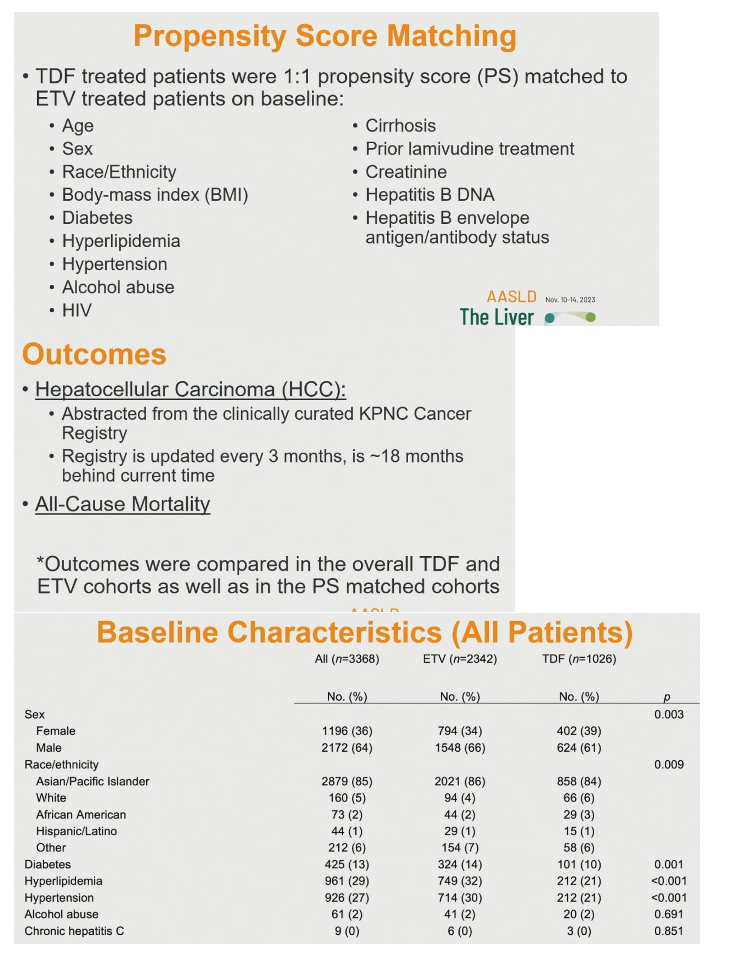
The investigators used propensity score matching to match each TDF-treated person to an ETV-treated person by age, sex, race/ethnicity, body mass index, diabetes, cirrhosis, prior lamivudine (3TC), hepatitis B DNA, hepatitis B envelope antigen/antibody status, and other variables detailed below. They compared rates of two outcomes-HCC diagnosis and all-cause mortality-for the overall TDF and ETV cohorts and for the propensity score-matched cohorts.
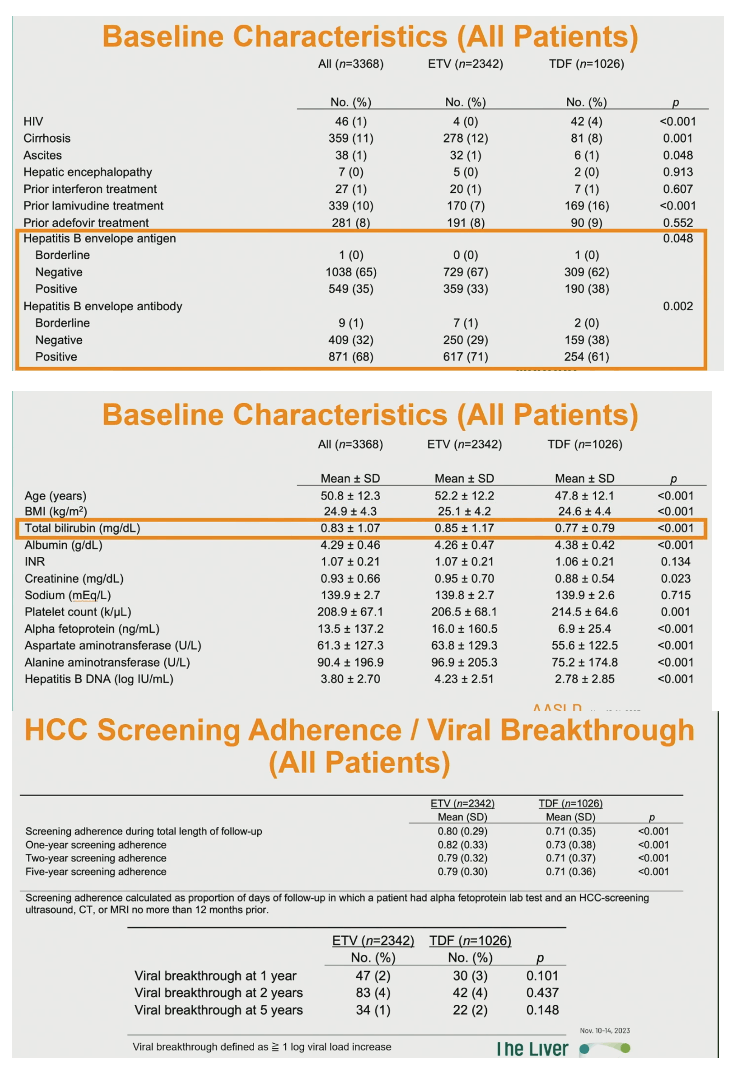
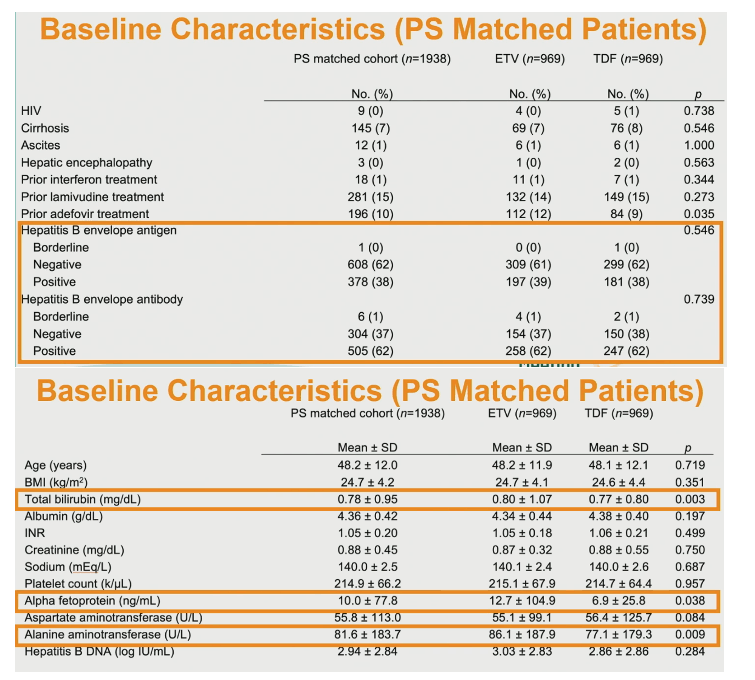
The analysis involved 2342 people taking ETV and 1026 taking TDF. The ETV group had a slightly but significantly higher proportion of males (66% vs 61%) than the TDF group (P = 0.003). The ETV and TDF groups also differed slightly but significantly in race/ethnicity (Asian/Pacific Islanders 86% vs 84%, whites 4% vs 6%, blacks 2% vs 3%, P = 0.009). And higher proportions prescribed ETV had diabetes (14% vs 10%, P = 0.001), hyperlipidemia (32% vs 21%, P < 0.001), and hypertension (30% vs 21%, P < 0.001). People taking ETV had a lower proportion with HIV infection (0% vs 4%, P < 0.001), a higher proportion with cirrhosis (12% vs 8%, P = 0.001), and a lower proportion with 3TC experience (7% vs 16%, P < 0.001). Only 2% in each group had alcohol abuse in their charts.
A lower proportion taking ETV than TDF was positive for hepatitis B envelope antigen (33% vs 38%, P = 0.048), while a higher proportion prescribed ETV was positive for hepatitis B envelope antibody (71% vs 61%, P = 0.002).
ETV takers were older than TDF takers (average 52.2 vs 47.8, P < 0.001), had a higher body mass index (25.1 vs 24.6 kg/m2, P < 0.001), and had higher hepatitis B DNA (4.23 vs 2.78 log IU/mL, P < 0.001), higher alanine aminotransferase, higher aspartate aminotransferase, higher alpha fetoprotein, higher creatinine, higher total bilirubin, lower albumin, and lower platelet count.
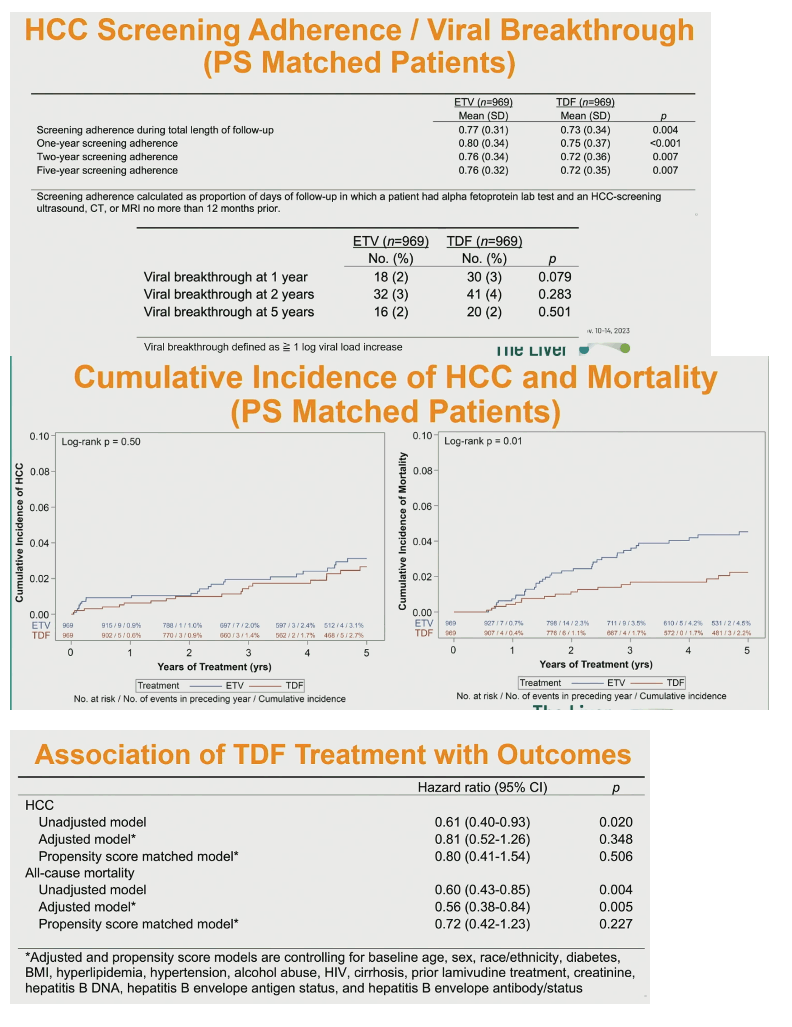
One-, 2-, and 5-year HCC screening adherence was significantly higher in the ETV group, as was screening throughout follow-up (0.80 vs 0.71, P < 0.001). Viral breakthrough rate at 1, 2, and 5 years did not differ significantly between the two groups.
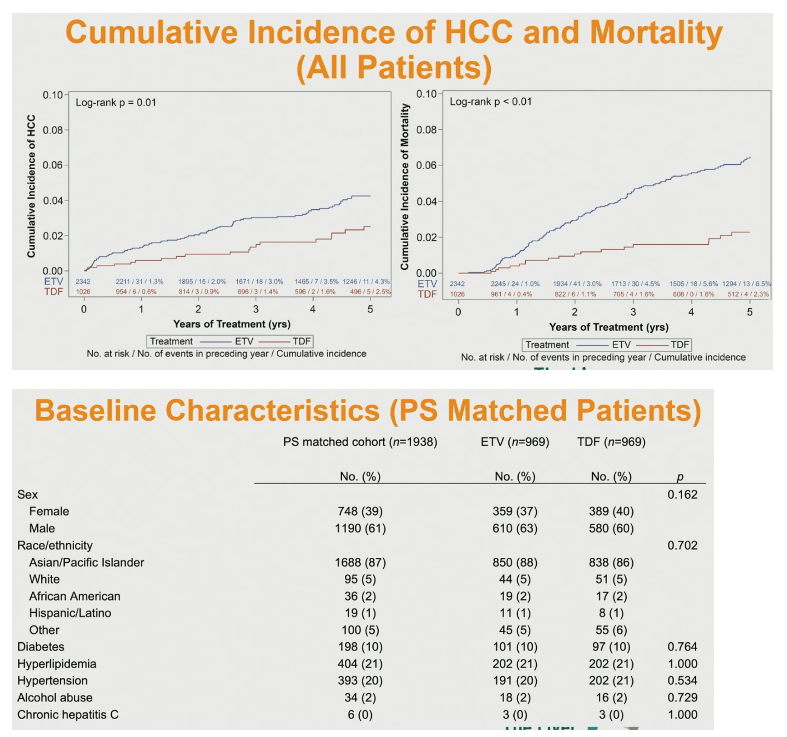
For the overall (unmatched) study group, cumulative incidence of HCC (P = 0.01) and all-cause mortality (P < 0.01) through 5 years were significantly higher in people taking ETV.
Propensity score matching involved 969 people taking ETV and 969 taking to TDF. They were well matched by sex, age, race/ethnicity, body mass index, HBV DNA, and history of diabetes, hyperlipidemia, hypertension, chronic HCV infection, alcohol abuse, HIV infection, cirrhosis, ascites, hepatic encephalopathy, prior interferon, prior 3TC, prior adefovir, and hepatitis B surface antigen positive and negative. Proportions positive and negative for hepatitis B surface antibody differed marginally but significantly between the groups (4% positive for ETV, 1% positive for TDF, P = 0.026).
Among these matched people, HCC screening adherence proved better in the ETV group than the TDF group (0.77 vs 0.73 throughout follow-up, P = 0.004). But viral breakthrough rate did not differ significantly between groups at 1, 2, or 5 years.
In this propensity score-matched population, cumulative incidence of HCC through 5 years no longer differed significantly between the ETV and TDF contingents, but 5-year all-cause mortality remained significantly higher in people taking ETV (P = 0.01).
Unadjusted models showed significantly lower risk of HCC (hazard ratio [HR] 0.61, 95% confidence interval [CI] 0.40 to 0.93, P = 0.020) and all-cause mortality (HR 0.60, 95 CI 0.43 to 0.85, P = 0.004) with TDF. A model adjusted for age, sex, race/ethnicity, body mass index, and an array of clinical complications found no advantage for TDF in preventing HCC. But TDF still held an edge in all-cause mortality (HR 0.56, 95% CI 0.38 to 0.84, P = 0.005). In the propensity score-matched model, however, TDF lost its advantage for the HCC outcome (HR 0.80, 95% CI 0.41 to 1.54, P = 0.506) and all-cause mortality (HR 0.72, 95% CI 0.42 to 1.23, P = 0.227).
The Kaiser team concluded that the apparent advantage of TDF over ETV in reducing risk of HCC and mortality probably reflects (1) provider prescribing patterns that favor ETV over TDF in higher-risk individuals and (2) better HCC screening adherence in people taking ETV. Because TDF and ETV did not differ in HCC and mortality outcomes in the propensity score-matched multivariate analysis, the researchers concluded that the drugs should be kept "on equal footing in guidelines as front-line chronic hepatitis B treatment options."
References
1. Seo SI, Stram DA, Chai KP, et al. Tenofovir versus entecavir in preventing HCC and death: answer from a large integrated health plan population. AASLD 2023, The Liver Meeting, November 10-14, 2023, Boston.
2. Choi WM, Choi J, Lim YS. Effects of tenofovir vs entecavir on risk of hepatocellular carcinoma in patients with chronic HBV infection: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021; 19:246-258.e9. doi: 10.1016/j.cgh.2020.05.008. https://pubmed.ncbi.nlm.nih.gov/32407970/

|
| |
|
 |
 |
|
|