 |
 |
 |
| |
Continued TDF for High HBV Load and Mild ALT Elevation Improves Fibrosis
|
| |
| |
AASLD 2023, The Liver Meeting, November 10-14, 2023, Boston
Mark Mascolini
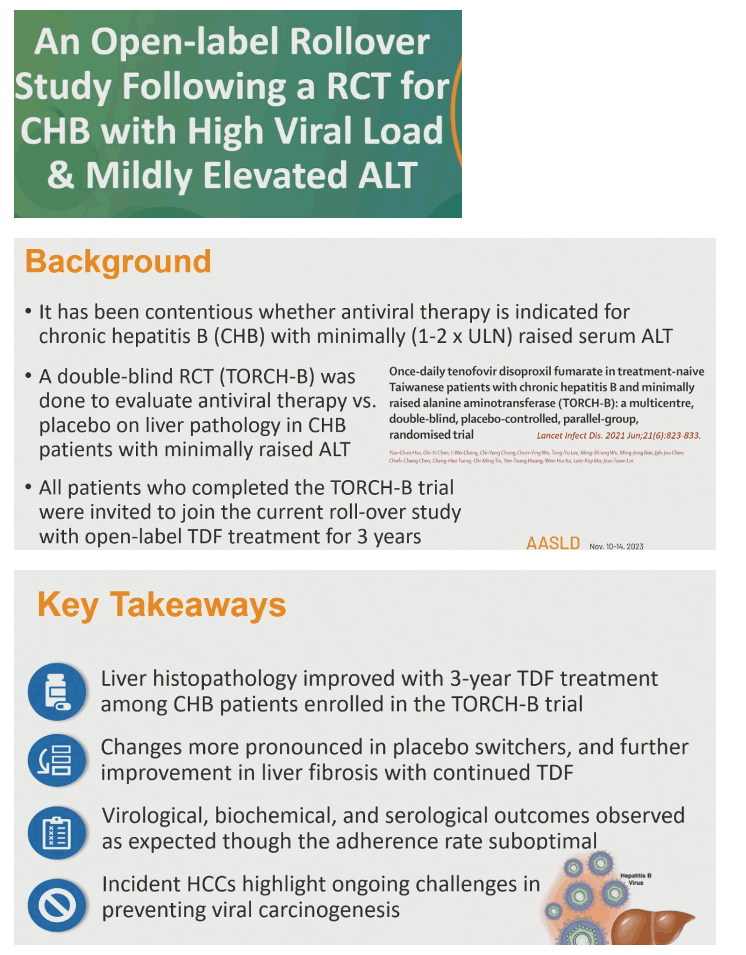
Continuing tenofovir disoproxil fumarate (TDF) for 3 years after a 3-year placebo-controlled trial further improved liver fibrosis in people with a high HBV load but only mildly elevated alanine aminotransferase (ALT) [1]. Yao-Chun Hsu from Taiwan's E-Da Hospital and colleagues from other centers reported that more than 80% of participants attained viral remission (undetectable HBV DNA) in this open-label rollover study.
At six teaching hospitals in Taiwan, TORCH-B, a double-blind placebo-controlled trial for people with serum HBV DNA above 2000 IU/mL and ALT above 1-fold but below 2-fold the upper limit of normal, found that TDF for 3 years halved the risk of 1-stage fibrosis progression but had little impact on necroinflammation (liver cell death and inflammation) [2].
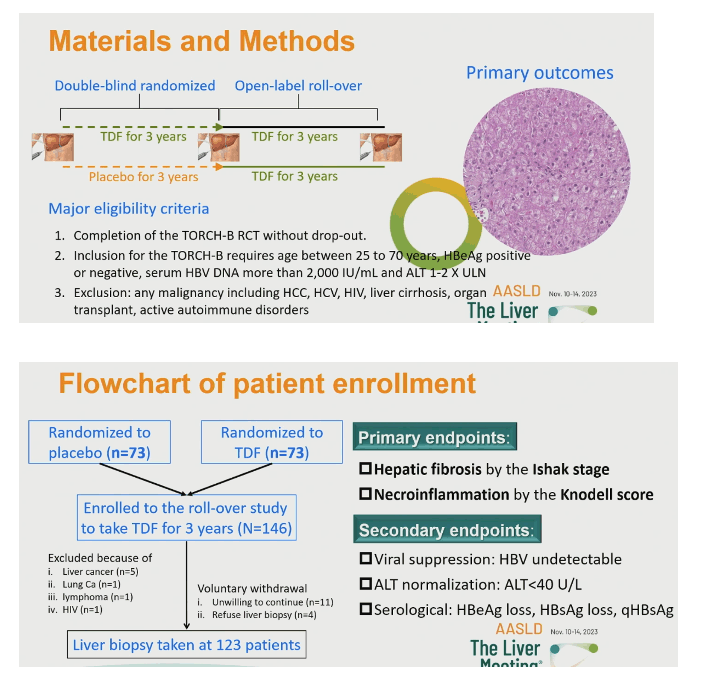
Everyone who completed TORCH-B could enter the rollover study, which aimed to treat all participants with TDF for the next 3 years [1,3]. Participants had to be between 25 and 70 years old when they entered TORCH-B and could be HBeAg-positive or negative. The rollover excluded people with any cancer including liver cancer, cirrhosis, HCV or HIV infection, organ transplant, or an active autoimmune disorder. Among the 146 people enrolled in the rollover, the researchers excluded 5 because of liver cancer and 1 each for lung cancer, lymphoma, or HIV infection. Eleven individuals were unwilling to continue, and 4 declined a rollover baseline biopsy. Ultimately, 123 people had a rollover baseline liver biopsy and a 3-year biopsy.
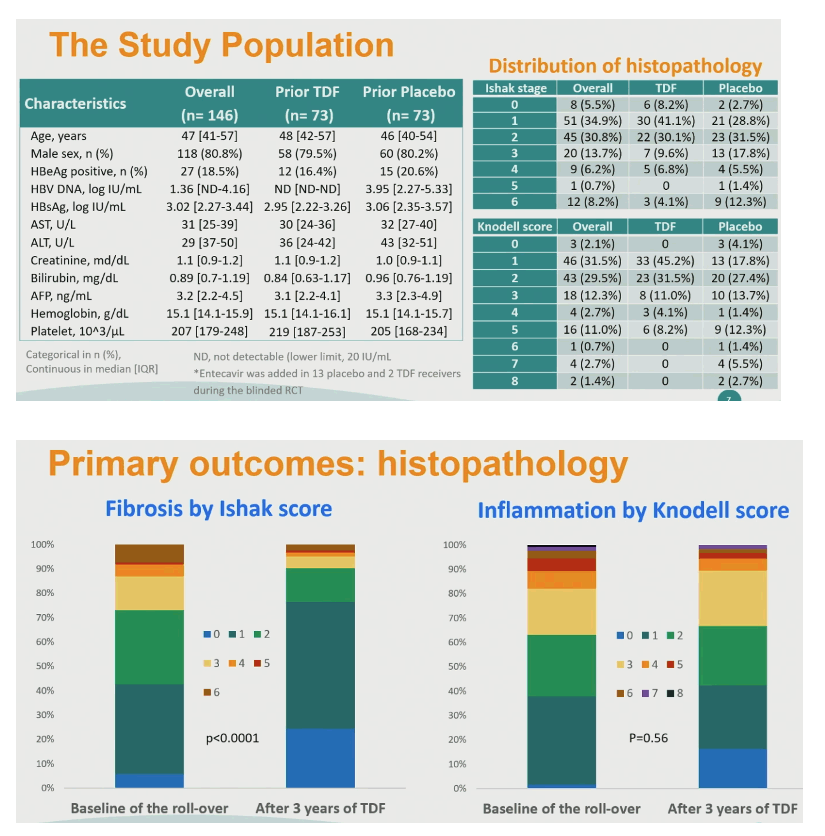
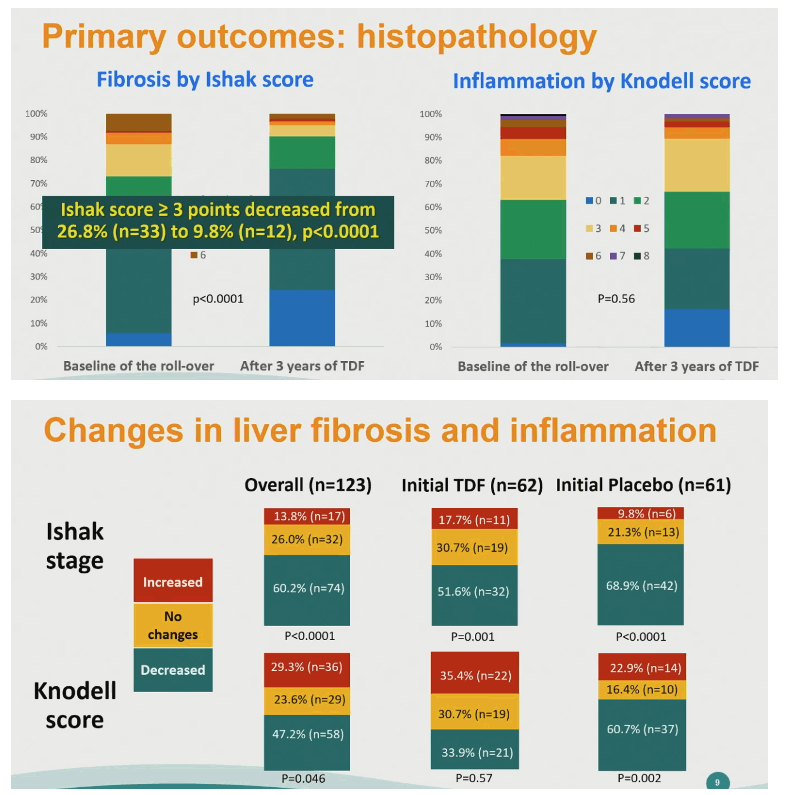
The 146 initial participants had a median age of 47, 81% were men, 18.5% were HBeAg-positive, median HBV load stood at 1.36 log IU/mL, and median ALT at 29 U/L. Among these 146 people, 42 (29%) were at Ishak stage 3 to 6 and 45 (31%) had a Knodell score of 3 to 8.
The proportion of 123 rollover completers with an Ishak stage of 3 to 6 fell from 26.8% (n = 33) at rollover baseline to 9.8% (n = 12) after 3 years of continued TDF, a significant drop (P < 0.0001). The overall proportion with a Knodell score of 3 to 8 changed little in 3 years. But Knodell score did improve significantly over 3 years in 61 people randomized to placebo in TORCH-B (P = 0.002).
Among people initially randomized to placebo versus TDF, Ishak score fell (improved) in 68.9% versus 51.6% in the rollover phase. For the initial placebo group versus the initial TDF group, Knodell score dropped (improved) in 60.7% versus 33.9% during the 3-year rollover.
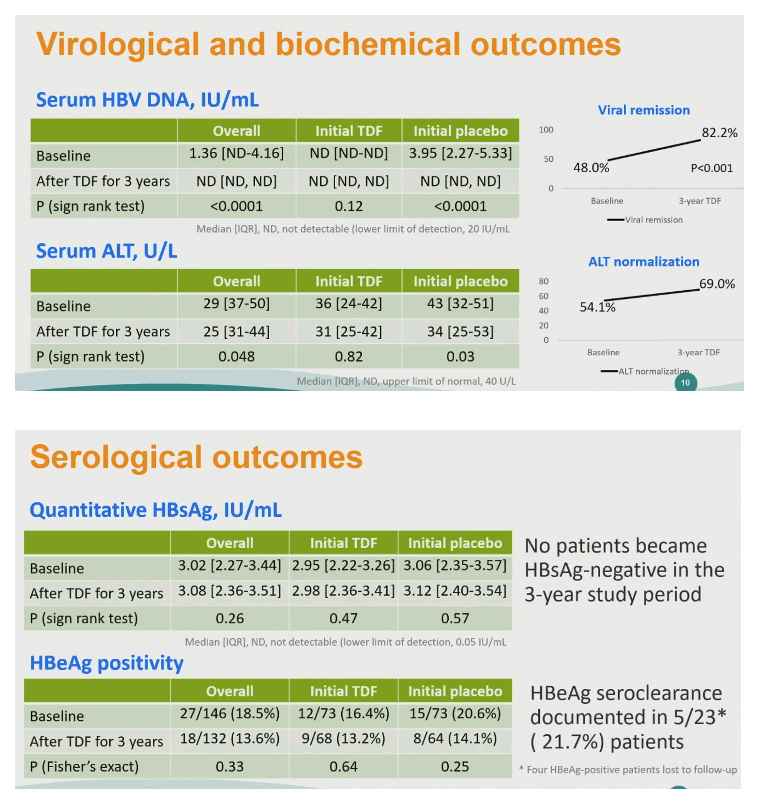
The proportion of people with undetectable HBV DNA rose significantly from 48.0% at rollover baseline to 82.2% after 3 more years of TDF (P < 0.001). The proportion with normal ALT climbed nonsignificantly from 54.1% to 69.0%. No one became HBsAg-negative during the 3-year study. The researchers documented HBeAg seroclearance in 5 of 23 people (21.7%).
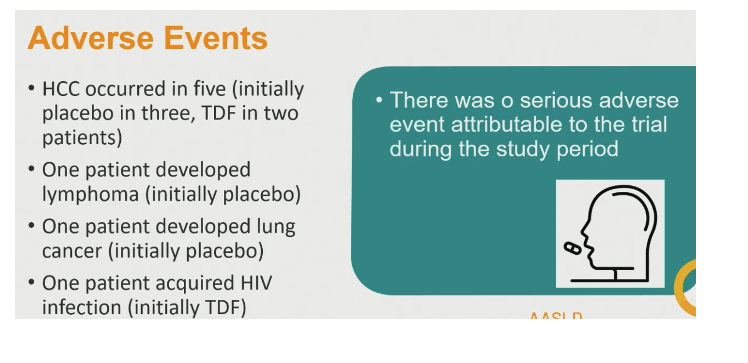
No serious adverse events could be attributed to the trial. Hepatocellular carcinoma got diagnosed in 3 people initially randomized to placebo and 2 randomized to TDF. Lymphoma developed in 1 person initially randomized to placebo and lung cancer in 1 randomized to placebo.
The researchers concluded that liver histopathology improved with TDF in a 3-year rollover from the TORCH-B trial, especially in those who took placebo in TORCH-B. But further improvement in fibrosis could also be seen in people who took TDF for 3 years in TORCH-B.
References
1. Hsu YC, Chen CY, Tseng CH, et al. An open-label rollover study following a randomized trial for chronic hepatitis B with high serum viral load but mild elevated aminotransferase. AASLD 2023, The Liver Meeting, November 10-14, 2023, Boston.
2. Hsu YC, Chen CY, Chang IW, et al. Once-daily tenofovir disoproxil fumarate in treatment-naive Taiwanese patients with chronic hepatitis B and minimally raised alanine aminotransferase (TORCH-B): a multicentre, double-blind, placebo-controlled, parallel-group, randomised trial. Lancet Infect Dis. 2021;21:823-833. doi: 10.1016/S1473-3099(20)30692-7. https://pubmed.ncbi.nlm.nih.gov/33524314/
3. ClinicalTrials.gov. Roll-over after 3-year trial for tenofovir in mild chronic hepatitis B. ClinicalTrials.gov ID NCT02463019. https://clinicaltrials.gov/study/NCT02463019
|
| |
|
 |
 |
|
|