 |
 |
 |
| |
DoxyPEP and Meningococcal Vax Keep Protecting MSM PrEP Users From STIs
|
| |
| |
30th CROI, Conference on Retroviruses and Opportunistic Infections, February 19-22, 2023, Seattle
Mark Mascolini
Doxycycline postexposure prophylaxis (DoxyPEP) and, separately, a meningococcal vaccine (4CMenB) continued to cut risk of sexually transmitted infections (STIs) in men who have sex with men (MSM) using preexposure prophylaxis (PrEP) who continued the preventive measures after preliminary reports of success in the DOXYVAC trial [1]. But French ANRS researchers cautioned that there is no magic bullet to prevent STIs in MSM using PrEP.
Some groups of PrEP users have exceedingly high rates of new STIs, for example, a 75.8 per 100 person-years cumulative incidence among MSM and transgender women in the ANRS Prevenir study [2]. DOXYVAC is a 2 x 2 factorial unblinded multicenter phase 3 trial aiming to show the superiority of DoxyPEP or 4CMenB over no doxycycline or no vaccination in MSM using PrEP [3].
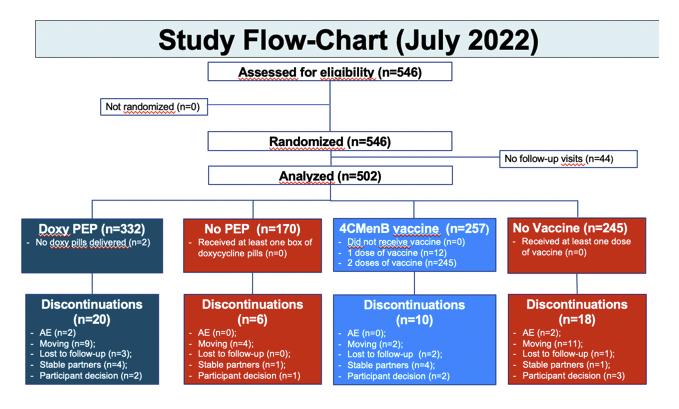
The trial enrolled 720 MSM taking PrEP for more than 6 months in the ANRS Prevenir study with a bacterial STI in the past 12 months but no STI symptoms. Jean-Michel Molina and ANRS colleagues randomized them in a 2-to-1 ratio to DoxyPEP or no PEP and in a 1-to-1 ration to 4CMenB vaccination or no vaccine. Primary efficacy endpoints were (1) the impact of DoxyPEP on time to first syphilis or chlamydia and (2) impact of 4CMenB on time to first N gonorrhoae infection.
Interim results in August 2022 indicated that DoxyPEP cut the rate of new STIs 65% (80% for chlamydia and syphilis and 55% for gonorrhea) [4]. A month after researchers reported those results, the trial Data and Safety Monitoring Board (DSMB) asked for an unblinded analysis of participants enrolled from January 2021 to July 2022. Because both DoxyPEP and the vaccine significantly lowered STI rates, the DSMB recommended stopping trial enrollment and offering the preventive interventions to all participants through a final visit in January 2023.
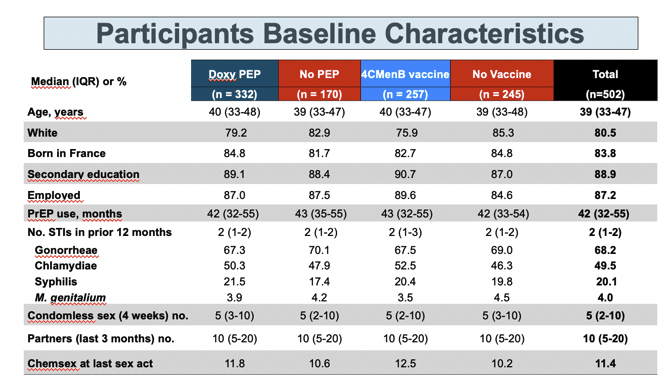
The analysis presented at CROI [1] involved 332 men randomized to DoxyPEP versus 170 to no PEP, and 257 men randomized to 4CMenB versus 245 to no vaccine. The total group had a median age of 39 years, 80.5% were white, 83.8% were born in France, and 87.2% had a job. The group had used PrEP for a median of 42 months and had a median of 2 STIs in the 12 months before entering the trial and a median of 5 condomless sex acts in the past 4 weeks.
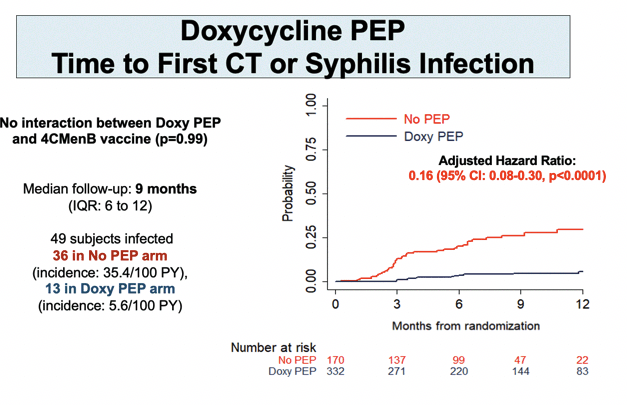
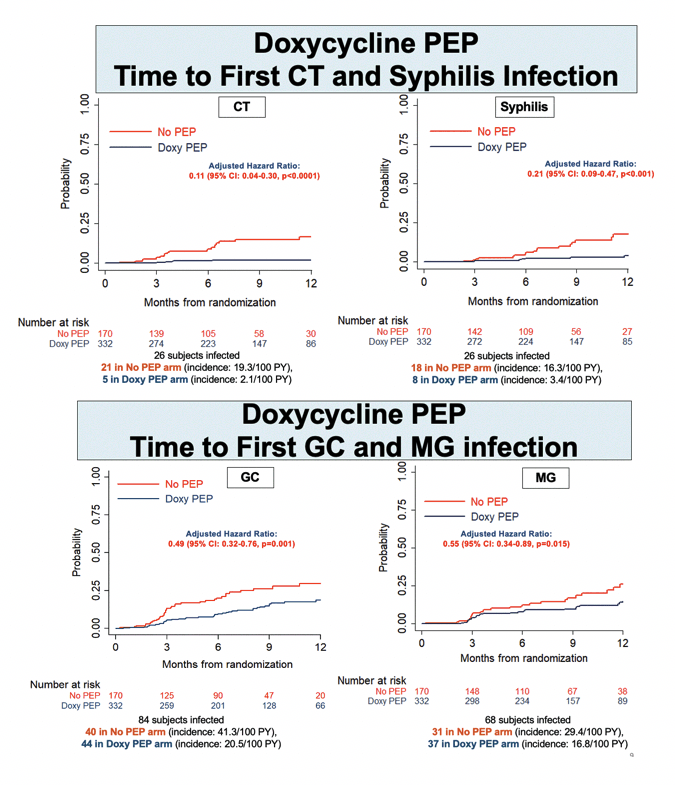
Through a median follow-up of 9 months (interquartile range [IQR] 6 to 12), 36 people in the no-PEP arm versus 13 in the DoxyPEP arm had a first syphilis infection to yield an 84% lower adjusted hazard ratio (aHR) with DoxyPEP (0.16, 95% confidence interval [CI] 0.08 to 0.30, P < 0.0001). Risk of first chlamydia infection was almost 90% lower with DoxyPEP than no PEP (aHR 0.11, 95% CI 0.04 to 0.30, P < 0.0001). DoxyPEP halved risk of first gonococcal infection (aHR 0.49, 95% CI 0.32 to 0.76, P < 0.001) and first meningococcal infection (aHR 0.55, 95% CI 0.34 to 0.89, P = 0.015).
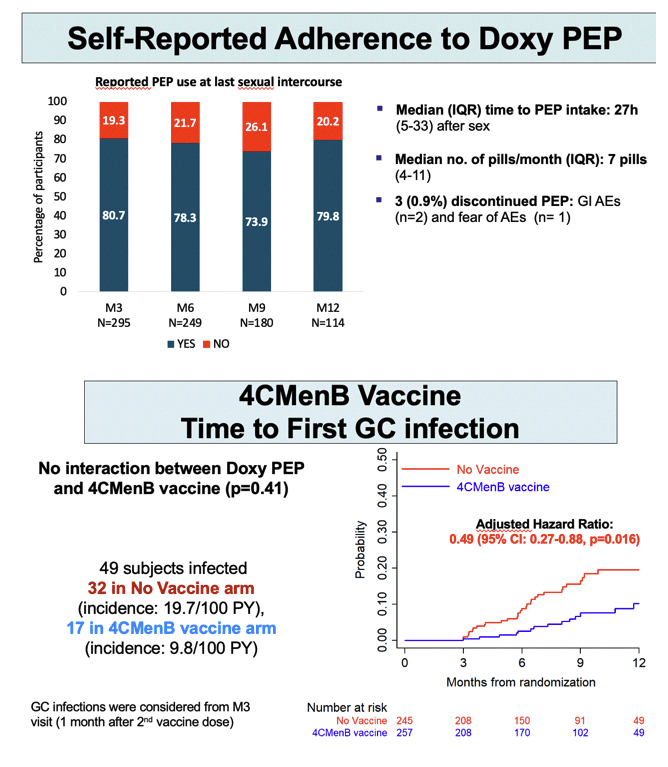
In the group randomized to DoxyPEP, median time to using PEP after sex stood at 27 hours (IQR 5 to 33), and a median 83% of participants (IQR 50 to 100) used PEP during following up. Proportions of men who used PEP the last time they had sex stayed about the same-between 70% and 80%-at trial months 3, 6, 9, and 12.
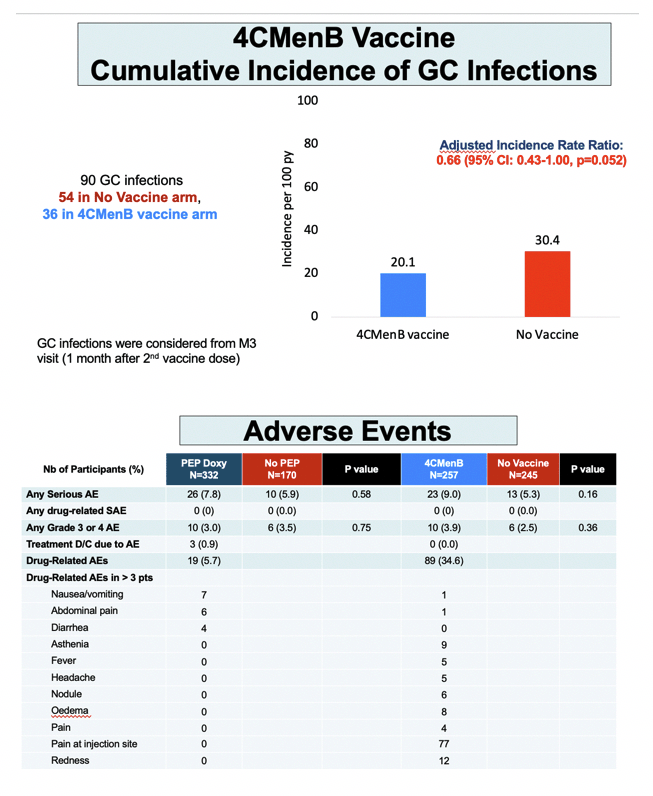
Getting the 4CMenB vaccine halved risk of first gonococcal infection versus no vaccine (aHR 0.49, 95% CI 0.27 to 0.88, P = 0.016). Gonococcal incidence worked out to 9.8 per 100 person-years in the vaccine arm (17 infections) and 19.7 per 100 person-years (32 infections) in the no-vaccine arm. Cumulative adjusted incidence rate ratio for gonococcal infection measured 20.1 per 100 person-years with the vaccine and 30.4 without the vaccine-which works out to a one third lower risk with 4CMenB (0.66, 95% CI 0.43 to 1.00, P = 0.052).
Further analysis found no interaction between DoxyPEP and 4CMenB in preventing STIs.
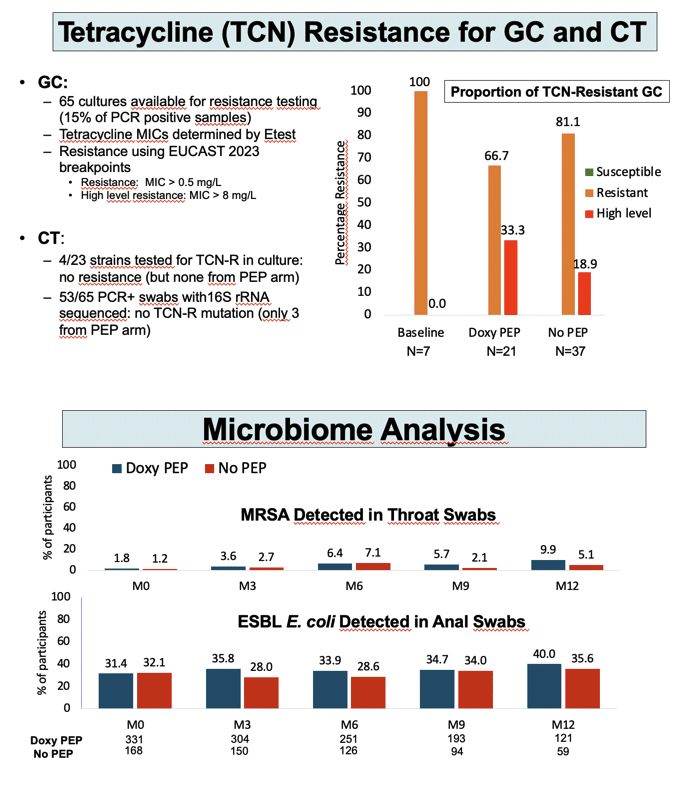
No one in any of the four study arms had a drug-related serious adverse event. Rates of discontinuation for an adverse event were 0.9% in the DoxyPEP group 0% in the 4CMenB group, and rates of any drug-related adverse event stood at 5.7% with DoxyPEP and 34.6% with 4CMenB. With DoxyPEP the most frequent adverse events were nausea or vomiting (7 of 332 men), abdominal pain (6 men), and diarrhea (4 men). Aside from injection site pain in 77 of 257 men getting the 4CMenB vaccine, the most frequent adverse events in vaccinated people were redness in 12, asthenia in 9, edema in 8, and nodules in 6.
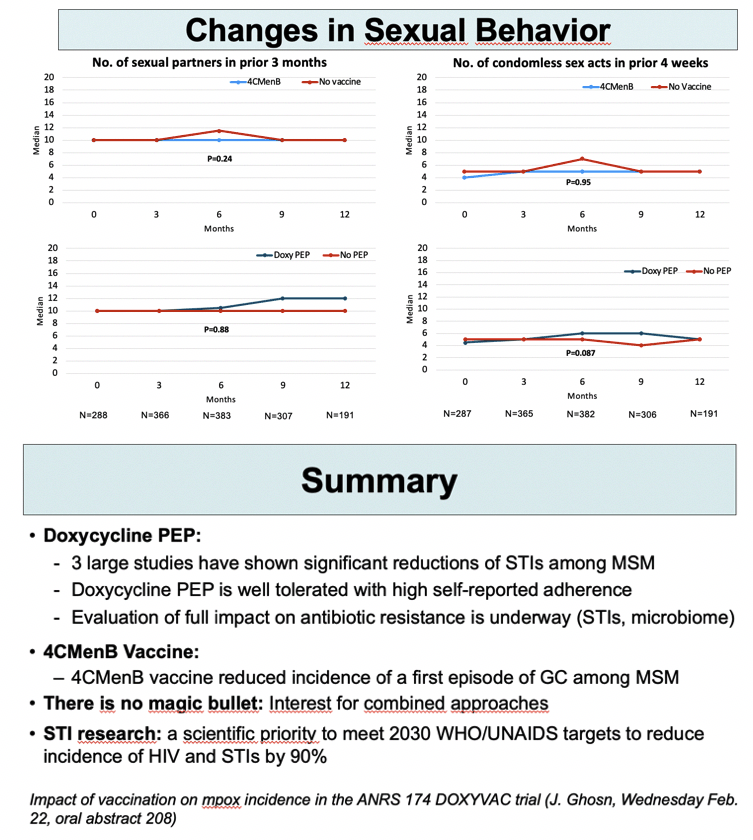
Overall and condomless sexual frequency did not change significantly through 12 months.
The ANRS investigators pointed out that 3 large studies now show significant drops in STI rates with DoxyPEP in MSM. The 4CMenB vaccine cut gonococcal infection rates in this trial. The researchers urged continued study of combined prevention approaches in people aiming to prevent HIV infection with PrEP because “there is no magic bullet.”
References
1. Molina JM, Bercot B, Assoumou A, et al. ANRS 174 DOXYVAC: an open-label randomized trial to prevent STIs in MSM on PrEP. 30th CROI, Conference on Retroviruses and Opportunistic Infections, February 19-22, 2023, Seattle. Abstract 119.
2. Molina JM, Ghosn J, Assoumou L, et al. Daily and on-demand HIV pre-exposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): a prospective observational cohort study. Lancet HIV. 2022;9:e554-e562. doi: 10.1016/S2352-3018(22)00133-3. https://pubmed.ncbi.nlm.nih.gov/35772417/
3. ClinicalTrials.gov. Combined prevention of sexually transmitted infections (STIs) in men who have sex with men and using oral tenofovir disoproxil fumarate/ emtricitabine (TDF/FTC) for HIV pre-exposure prophylaxis (PrEP) (DOXYVAC). ClinicalTrials.gov identifier NCT04597424. https://clinicaltrials.gov/ct2/show/NCT04597424
4. NATAP. Doxycycline PEP cuts bacterial STI rate over 60% after condomless sex. AIDS 2022, July 29-August 2, Montreal. Abstract OALBX0103. https://www.natap.org/2022/IAC/IAC_24.htm

|
| |
|
 |
 |
|
|