 |
 |
 |
| |
Brain Aging Gap was significantly elevated in PWH compared to PWoH (p=0.001).
|
| |
| |
Download the PDF here
Brain Aging is Worse in PWH: HCV 4 yrs added brain aging gap, CVD 10 yrs added brain aging gap - cd4, viral load & non-HIV drivers of health such as comorbid conditions & socioeconomic status are important
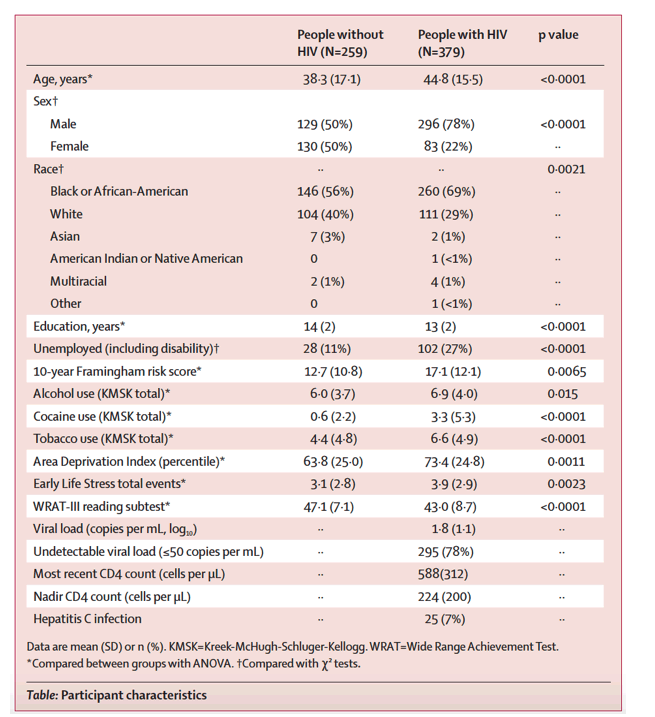
Effects of clinical, comorbid, and social determinants of health on brain ageing in people with and without HIV: a retrospective case-control study.
February 07, 2023
Lancet HIV
Petersen et al.
People with HIV have increased average brain-age gap relative to seronegative peers; however, available data indicate that within-group variability in brain ageing exceeds between-group differences, and accounting for heterogeneity is crucial.12,14
Taken together, these results paint a nuanced picture of ageing with HIV. Traditional clinical variables such as viral load and T-cell counts affect neuropathology; however, non-HIV drivers of health such as comorbid diseases and socioeconomic status are growing in importance. Together, such factors could account for heterogeneity in neurocognitive outcomes in older people with HIV and people without HIV. Identification of brain-ageing correlates could lead to a broadened perspective on health for people ageing with chronic infectious disease while navigating
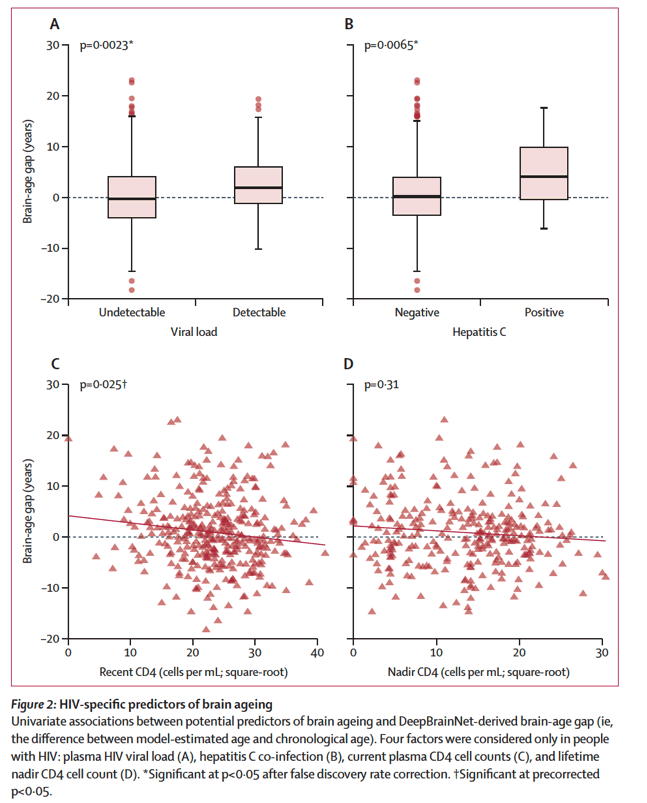
The first group of factors that could affect brain ageing are direct effects of HIV. We examined four key variables: viral load, current CD4 lymphocytes, nadir CD4 count, and hepatitis C co-infection. Detectable viral load was significantly associated with elevated brain-age gap, consistent with a large literature implicating viral suppression and immune re-constitution in preserved neurocognitive function.26 Hepatitis C was associated with approximately 4 years of added brain-age gap in people with HIV, suggesting that the pathological effects of HIV and hepatitis C have additive effects on brain health.27 Thus, achieving control of both viruses is likely to be important for healthy brain ageing.
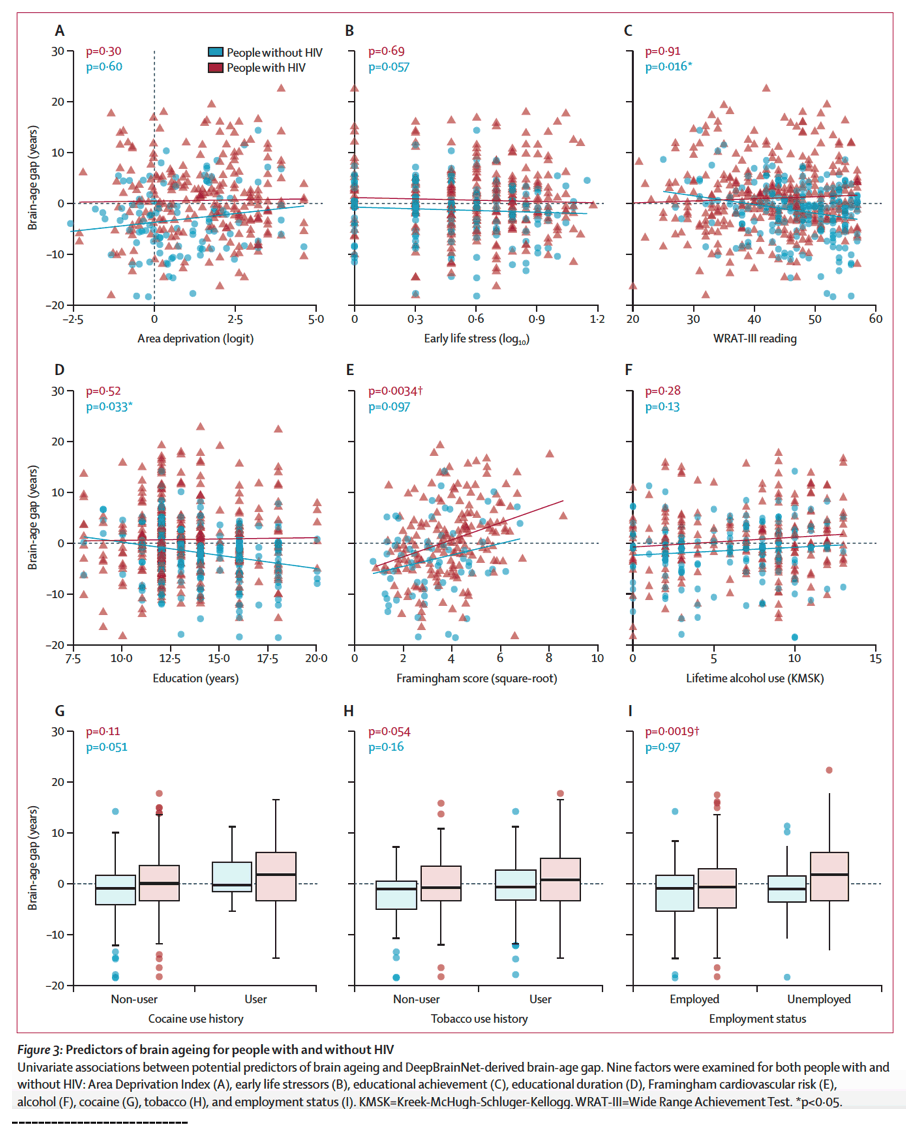
Substance use disorders are also more prevalent among people with HIV than among the general population, and the effects of a history of drug misuse must be considered when studying neurocognitive deficits.29 Previous work has linked drug use with brain structural and functional changes in people with HIV, but associations with brain-age gap have not been characterised. In multivariate analysis of people without HIV, we found a positive association between brain-age gap and alcohol use, potentially indicating that neurotoxic effects of heavy consumption influence MRI-based brain age. The absence of a similar effect in people with HIV could be a function of the colinearity between alcohol use and other factors (eg, cardiovascular disease) for which stronger links were found.
The strongest and most consistent brain-age gap association was with Framingham cardiovascular risk score. The modelled difference in brain-age gap between individuals at minimum (<2%) and maximum (>60%) cardiovascular risk in this study was over 10 years. In univariate modelling, this association was significant in people with HIV; however, the effect size was similar in people without HIV, marking cardiovascular disease as a good candidate for a general brain ageing risk factor. However, it remains especially relevant for people with HIV who have increased vascular disease compared with the general population.28 These findings suggest that maintenance of normal blood pressure and cholesterol could be crucial for people with HIV who have established viral control but remain vulnerable to cardiovascular disease.
One unexpected finding was the detection of a protective effect of educational achievement in people without HIV alone, in contrast with years of formal education, which showed no significant association. The Wide Range Achievement Test reading score had a significant negative correlation with brain-age gap in multivariate analysis, such that for each point of improvement on the Wide Range Achievement Test, the mean brain-predicted age was reduced by 0⋅45 years. The apparent absence of this effect in people with HIV is challenging to interpret but might indicate that the enhanced cognitive reserve conferred by quality of education might not be fully realised in people with HIV who experience clinical and social stressors related to lower rungs on the hierarchy of needs (ie, those related to safety, food security, or other basic needs).
Social determinants of health were given consideration in this study as economic instability and social marginalisation disproportionately affect people with HIV. In addition to education, we examined three major social factors: childhood stress, residential neighbour-hood quality from geospatially derived Area Deprivation Index, and unemployment status. Although neither the Early Life Stress Questionnaire or Area Deprivation Index were associated with brain-age gap, Area Deprivation Index had positive associations with brain-age gap in the combined cohort model. Finally, unemployment status showed a strong linkage with increased brain-age gap in people with HIV, although causality remains unclear because neurocognitive impairment associated with accelerated brain ageing might precede loss of employment.
Anatomically, the brain-age gap was interpreted by correlation with FreeSurfer volumes. Although this approach does not capture all the complex patterns identified by the neural network, it provides an approximation of relevant features. Results were congruent with literature on brain structure and ageing: positive associations with brain-age gap were confined to CSF compartments and T1 white matter hypointensities, whereas the strongest negative correlations were in subcortical structures that atrophy with age, particularly amygdala, hippocampus, and corpus callosum.30 These results suggest that DeepBrainNet identifies ageing-relevant imaging features.
By use of neuroimaging, machine learning, and model selection, we have shown that a combination of clinical measures, comorbidities, and social determinants of health are associated with brain-predicted age in people with HIV and people without HIV. Cardiovascular disease burden, detectable HIV viral load, and hepatitis C co-infection were identified as the strongest univariate correlates of brain-age gap in people with HIV. Additionally, the effects of social factors such as unemployment and area socioeconomic deprivation were identified in multivariate regression. Differences in significant variables between univariate and multivariate analyses could have several causes. For example, two predictors with high colinearity, accounting for shared variance in the response variable, could both show significant effects on brain-age gap in independent univariate tests, but not in a multivariate model. Finally, modelling people with HIV and people without HIV together implicated Framingham score, alcohol use, Area Deprivation Index, unemployment, male sex, and hepatitis C with older-appearing brain phenotypes. Notably, HIV itself was not significantly associated with brain-age gap when modelling these other factors, suggesting the relative importance of non-HIV drivers of brain ageing in the combination ART era.
Substantial evidence now implicates non-HIV risk and resilience factors in ageing effects for people with HIV.23,24 Health disparities between people with HIV and people without HIV partly reflect the legacy of early uncontrolled infection, but these residual effects alone are insufficient to explain the persistence of neurocognitive impairment among people with well controlled HIV.25 As a result, comorbidities and social determinants of health are increasingly salient features in people with HIV with suppressed viral loads, immune reconstitution, and the expectation of longevity.
Among HIV-specific variables (figure 2; appendix p 4), detectable viral load (p=0⋅0023) and hepatitis C co-infection (p=0⋅0065) were significantly positively associated with brain-age gap. CD4 cell count was negatively associated (p=0⋅025) but fell short of significance after false discovery rate adjustment.
The best model for people with HIV (figure 4) included Framingham score (p=0⋅0019; β=1⋅43), hepatitis C (p=0⋅073; β=3⋅90), and unemployment (p=0⋅020; β=3⋅21). The best model for people without HIV (figure 4) included alcohol use (p=0⋅0041; β=0⋅40), early life stress (p=0⋅047; β=-3⋅27) and Wide Range Achievement Test reading (p<0⋅0001; β=-0⋅304), with a non-significant term for unemployment (p=0⋅79; β=0⋅327). Finally, the best model for the combined cohort (people with HIV and people without HIV; figure 5) included Framingham score (p=0⋅0039; β=1⋅06), hepatitis C (p=0⋅037; β=3⋅84), Area Deprivation Index (p=0⋅033; β=0⋅684), and un employment (p=0⋅00010), with retained non-significant terms for male sex (p=0⋅078; β=2⋅11) and alcohol use (p=0⋅090; β=0⋅224).
---------------------------
CROI 2023 Abstract
Worse Brain Aging in PWH: factors - CVD, Social Determinants, HCV, Viral Load, Unemployment
These findings confirm the hypothesis that comorbid and socioeconomic factors are associated with brain aging alongside clinical metrics such as viral load. A broadened clinical perspective on healthy aging with HIV may require increased focus on such non-traditional determinants of health. Regional correlations between brain-age gap (BAG) and cortical or subcortical volumes.
The brain-age gap (BAG), defined as the difference between brain-predicted age and true chronological age, was modeled as a function of clinical, comorbid, and social factors for PWH and PWoH.
BAG was significantly elevated in PWH compared to PWoH (p=0.001).
HIV CLINICAL, COMORBID, AND SOCIAL DETERMINANTS OF HEALTH ARE LINKED WITH BRAIN AGING
CROI 2023 Feb 20-23
Kalen J. Petersen, Tina Lu, Julie Wisch, June Roman, Nicolas Metcalf, Sarah Cooley, Beau Ances
Washington University in St. Louis, St. Louis, MO, USA
STUDY AIM: Identify factors beyond serostatus or viral load that best account for variability in brain aging, individually & in concert.
Background: Neuroimaging reveals brain changes linked with HIV infection and neurocognitive disorders. However, group-level differences between persons with HIV (PWH) and persons without HIV (PWoH) conceal substantial within-group heterogeneity in risk factor exposures. PWH experience elevated comorbidities such as cardiovascular disease and socioeconomic deprivation. However, the contribution of such factors to brain aging in PWH and PWoH remains to be quantified.
HIV is thought accelerate biological aging in multiple tissues including CNS- Aung et al., AIDS Behav 2020.
Cognitive impairment affects a large minority of persons with HIV (PWH) even in cART era - Heaton et al., Neurology 2010.
Complexity of aging for PWH increased by multiple prevalent co-morbidities: substance use, social determinants CVD, co-infections - all affect brain health.
Methods: PWH (n=379; age=44.8±15.5 yr.; 78.1% biologically male; 68.6% African-American; 77.8% undetectable viral load [< 50 copies/mL]) and PWoH (n=259; age=38.3±17.1 yr.; 49.8% male; 56.4% A.A.) were clinically characterized and underwent 3-Tesla T1-weighted magnetic resonance imaging (MRI).
DeepBrainNet, a publicly available machine learning algorithm, was applied to estimate brain-predicted age from MRI.
The brain-age gap (BAG), defined as the difference between brain-predicted age and true chronological age, was modeled as a function of clinical, comorbid, and social factors for PWH and PWoH separately using linear regression and variable selection.
To identify spatial patterns relevant to pathological aging, BAG values were correlated with regional brain volumes quantified with FreeSurfer v5.3.
Results:
BAG was significantly elevated in PWH compared to PWoH (p=0.001).
In PWH, BAG was positively associated with Framingham cardiovascular risk score (p=0.002), detectable viral load (p=0.006) and hepatitis C co-infection (p=0.006).
Over 10 years brain-age difference between minimum & maximum cardiovascular risk.
In PWH, hepatitis C-positive status was linked with older-appearing brain
Effect size = +4.2 years
Co-infection plays a known role in cognitive impairment
After variable selection, the model for PWH retained Framingham score and hepatitis C, and added early life stress and area deprivation index, a socioeconomic measure combining geospatial data on housing, employment, education, and income.
Employment: Unemployed or on-disability status associated with a greater brain-age gap in PWH only; Effect size: +3.1 years; Causality of this effect is unclear
SOCIAL DETERMINANTS of Health
Area Deprivation Index (ADI): is the aggregate measure of social determinants from tract-level data, reflects neighborhood socioeconomic factors (not participant specific): income, education, housing quality.
Elevated BAG was associated with reduced regional gray matter volumes and larger ventricles, with distinct spatial patterns by serostatus. PWH showed more negative BAG-volume associations in cortical regions such as precuneus and posterior cingulate gyrus, and subcortical structures including putamen, globus pallidus, and cerebellum (see Figure).
Conclusion: These findings confirm the hypothesis that comorbid and socioeconomic factors are associated with brain aging alongside clinical metrics such as viral load. A broadened clinical perspective on healthy aging with HIV may require increased focus on such non-traditional determinants of health. Regional correlations between brain-age gap (BAG) and cortical or subcortical volumes. There is a High variability in brain aging for both PWH & PWoH.
|
| |
|
 |
 |
|
|