 |
 |
 |
| |
Summary from CROI 2023 for HIV and liver disease
What is new in viral hepatitis coinfection?
Is fatty liver disease a growing concern?
New insights from CROI 2023
|
| |
| |
Jurgen K. Rockstroh M.D., Professor of Medicine
University of Bonn, Germany
Correspondence:
Prof. Dr. Jurgen K. Rockstroh
Department of Medicine I
University Hospital Bonn
Venusberg-Campus 1
53127 Bonn
Germany
Introduction
2023 marked the 30th CROI conference since the first meeting in Washington DC back in 1993 and was finally back as a mainly f-2-f meeting, which was long awaited for by CROI delegates to reconnect and discuss the newest findings in various areas of HIV research in person again. HIV and liver disease remained an important topic this year but received fewer abstract submissions than before the onset of the COVID-19 pandemic. As last year, the oral abstract session for hepatitis research was integrated into the session on tuberculosis and hepatitis. Indeed, there were only three oral hepatitis abstracts all on viral hepatitis B coinfection, one on translational HBV research (1), and two on clinical aspects of HBV management (2,3). In addition, there was an oral abstract presentation on shorter treatment durations for recently acquired HCV infection in the oral abstract session 12 on "Antiviral Strategies for Treatment and Preventions" (4) as well as a themed poster discussion on "Steatohepatitis: Sex Differences and other Risk Factors" (5-8). Moreover, there were numerous interesting abstract submissions, which focused on various aspects of liver disease in HIV and were part of several poster sessions (9-15). Finally, yet importantly one highlight which needs to be mentioned, was the excellent HBV plenary: "The Path to Hepatitis B Cure, which was given by Anna S. Lok on Monday morning.
The following summary aims at highlighting some of the exciting new data reported at CROI 2023 and to initiate a discussion about the impact of these new data on guidelines and clinical management of liver disease in HIV-coinfected individuals.
Hepatitis B
At this year CROI, a new analysis from the ongoing ALLIANCE study was presented (2). Aim of this phase-3-study (ALLIANCE) was to compare outcomes of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) vs dolutegravir + emtricitabine/tenofovir disoproxil fumarate (DTG+F/TDF) in participants initiating treatment for HIV-1 and HBV and to examine HBV DNA suppression and predictors of HBs/eAg loss in this coinfected population. Adults with HIV-1/HBV were randomized 1:1 to initiate blinded treatment with B/F/TAF or DTG+F/TDF (with corresponding placebo). Table 1 summarizes the main baseline characteristics of the study participants.
Table 1: Baseline characteristics of the ALLIANCE study
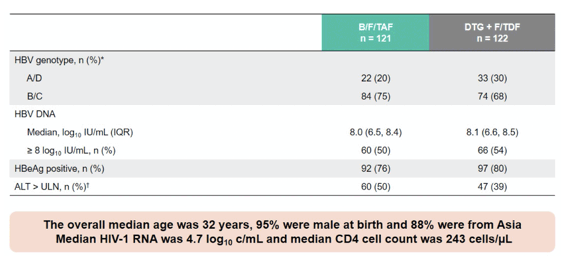
Co-primary endpoints were proportion with HIV-1 RNA < 50 copies/mL (Snapshot) and HBV DNA < 29 IU/mL. Subgroup analyses determined the proportion with HBV DNA < 29 IU/mL stratified by BL HBV DNA < or = 8 log10 IU/mL and HBeAg status (-/+ at BL). At IAS in Montreal in July 2022 a first data analysis was presented which demonstrated that adults with HIV-1/HBV-coinfection starting antiviral therapy, both B/F/TAF and DTG+F/TDF achieved high HIV-1 suppression at year 1, with B/F/TAF however, resulting in superior HBV-DNA suppression and significantly more HBeAg seroconversions (16). At CROI results from a multivariate analysis (MVA) were presented which evaluated predictors of HBV DNA < 29 IU/mL and HBs/eAg loss in the ALLIANCE study (2). Table 2 summarizes the outcome of that analysis.
Table 2: Baseline Predictors of HBV Treatment Response: Multivariate Logistic Regression Analysis (Full Analysis Set)
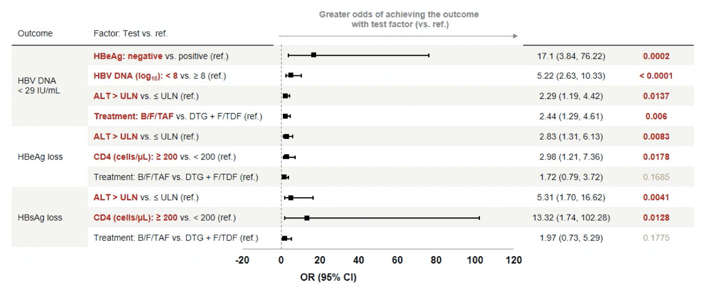
Of note, in adults with HIV/HBV coinfection initiating antiviral therapy for the first time, after 48 weeks, significantly more participants on B/F/TAF versus DTG + F/TDF had HBV DNA < 29 IU/mL, and normal ALT. The impact of treatment on HBeAg seroconversion rate however was no longer significant. Several baseline factors were determined to be predictors of HBV-DNA suppression, including B/F/TAF treatment, HBeAg-negative status, HBV DNA < 8 log10 and ALT > ULN at baseline. ALT > ULN and CD4 ≥ 200 cells/μL at baseline were predictors of HBeAg and HBsAg loss. Interestingly, trials in HBV mono-infection with much larger patient numbers, which compared TDF with TAF were not able to detect any differences between the two HBV therapies with regard to HBV suppression or HBeAg seroconversion. As HBeAg as well as HBsAg seroconversion occurs more frequently in people living with HIV coinfection, (also in the ALLIANCE study) the question arises whether there were potentially differences with regard to the baseline characteristics of both groups, which are known to affect HBV seroconversion rates. Indeed, there were slightly more individuals with higher baseline HBV-DNA levels in the TDF group (see table 1). This may also contribute to higher HBV undetectability rates in the TAF group. In addition, quantitative HBsAg levels were not provided for both groups. A longer follow-up is warranted to see whether these results persist over time. Overall, median HBV-DNA decline up to week 48 did not differ between the two groups making differences in antiviral potency over time rather unlikely.
The second HBV abstract presented in the oral hepatitis abstract session dealt with the PAGE-B risk score for hepatocellular cancer risk assessment (2). The corresponding risk score is used by EASL and EACS guidelines to select at risk candidates with chronic hepatitis B for HCC screening. The PAGE-B score, which integrates thrombocyte count, age and gender, however has never been validated in PLWH. Aim of this study was therefore to evaluate the external validity of PAGE-B to determine the HCC risk among individuals with HIV/HBV coinfection. PAGE-B represents a simple and reliable score for prediction of the 5-year HCC risk in Caucasian CHB patients under antiviral therapy. PLWH with a positive HBsAg and without HCC before starting tenofovir from four European cohorts were included and the predictive performance of PAGE-B on HCC occurrence over 15 years of tenofovir-containing antiretroviral therapy (ART) was assessed. In total, 2.963 individuals with HIV/HBV coinfection on tenofovir-containing ART were included. PAGE-B was <10 in 26.5%, 10-17 in 57.7%, and ≥18 in 15.7% of patients. Within a median follow-up of 9.6 years, HCC occurred in 68 individuals (2.58/1000 patient-years, 95% confidence interval [CI] 2.03-3.27). Figure 1 shows the association between PAGE-B distribution and hepatocellular carcinoma.
Figure 1: PAGE-B distribution and hepatocellular carcinoma
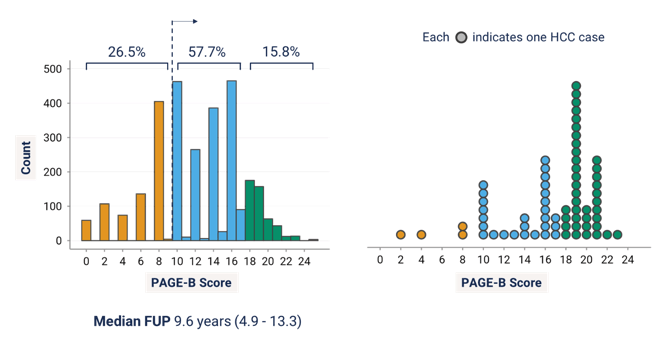
In this external validation study, the PAGE-B score showed good accuracy in predicting the HCC risk in a large collaboration of European cohorts of individuals living with HIV and HBV infection. Similar to the original derivation study, individuals with a score below 10 were at very low risk of HCC, with a negative predictive value above 99%, confirming the usefulness of PAGE-B to target HCC surveillance efforts in individuals with HIV/HBV coinfection (see figure 2).
Figure 2: Screening threshold
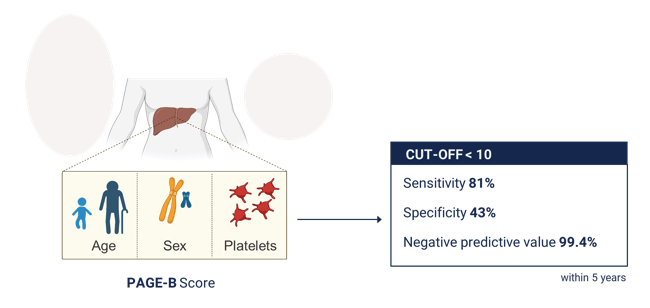
Of note, 3 of the patients with a low PAGE-B score and subsequent HCC development were from African origin. Clearly more data is needed in this particular subgroup to assess the validity of this score in a larger black population.
In the poster session an interesting poster was presented on the kinetics of HBV RNA and core-related-antigen levels in PLWH who achieved functional HBV cure (9). Of note, Hepatitis B core-related antigen (HBcrAg) and circulating HBV (hepatitis B virus) RNA correlate with intrahepatic covalently closed circular DNA (cccDNA) levels and cccDNA transcriptional activity. Therefore, both markers may help identify persons who eventually experience functional HBV cure on antiviral therapy. Aim of the study was to compare long-term kinetics of circulating HBV RNA and HBcrAg levels among persons living with HIV and HBV treated with tenofovir in the Swiss HIV Cohort Study. 29 participants with functional HBV cure and 29 participants without functional HBV cure on tenofovir-containing antiretroviral therapy were enrolled into this study. Participants in both groups were matched 1:1 for age (10-year caliber), sex, pretreatment with lamivudine and CD4+ T-cell count category. Table 3 summarizes the main characteristics of the two study groups.
Table 3: Characteristics of participants with and without functional HBV.
HBcrAg and HBV RNA levels were modelled over time using linear regression, while incorporating time as restricted cubic splines with five knots located at the 5th, 27.5th, 50th, 72.5th and 95th percentile. Functional HBV cure was defined as the occurrence of a quantitative hepatitis B surface antigen (qHBsAg) <0.05 IU/ml. Among participants with HBcrAg >3 log10 U/ml at tenofovir start, 14/22 (64%) with functional HBV cure compared to 8/25 (32%) without functional HBV cure had an HBcrAg decline of ≥1 log10 U/ml after 1 year (p=0.04,). After 5 years, 18/28 (64%) participants with functional HBV cure had HBcrAg ≤3 log10 U/ml compared to 6/26 (23%) without functional HBV cure (p=0.003). Among participants with detectable HBV RNA levels at tenofovir start, 14/17 (82%) with functional HBV cure compared to 10/18 (56%) without functional HBV cure had an HBV RNA decline of ≥1 log10 copies/ml 1 year after tenofovir start (p=0.15). After 5 years, HBV RNA was undetectable among 26/28 (93%) participants with functional HBV cure compared to 17/26 (65%) without functional HBV cure (p=0.02). In summary, a decline in circulating HBV RNA and HBcrAg levels during tenofovir therapy is more likely in PLWH with functional HBV cure than in those without cure. HBV RNA and HBcrAg indeed may be promising predictive markers of functional HBV cure.
Hepatitis D
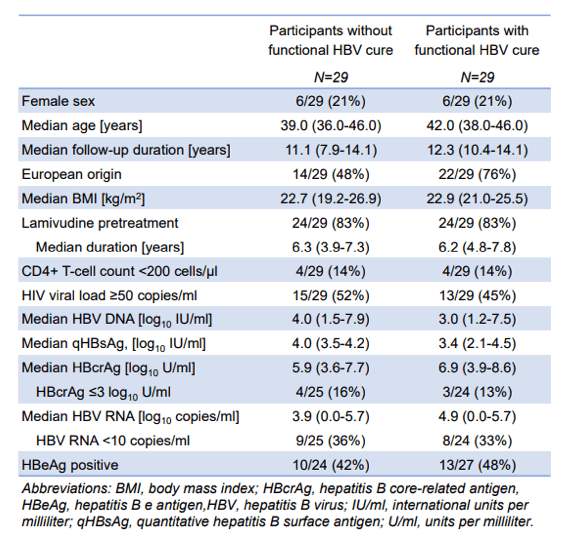
At this year CROI some of the first real-life treatment results with bulevirtide were presented from the French EAP (10). Aim of the study was to evaluate the efficacy and safety of bulevirtide (BLV) 2mg subcutaneous daily with or without PEG-IFN2a for 12 months in HIV co-infected patients treated in the French BLV early access program. Overall, 21 patients with HIV/HBV/HDV were enrolled in the BLV early access program with either compensated cirrhosis/severe fibrosis or moderate fibrosis with elevated ALT levels, Patients received BLV 2mg QD SC alone (N=14) or in combination with PEG-IFNα once weekly (N=7) according to physician's choice. Patient characteristics were as follows: male 71%, mean age 48 years, cirrhosis 52%, median HDV-RNA 6.09 log10IU/mL, median HIV-RNA 0 cp/mL (three patients had detectable HIV-RNA at day 0, all < 100 cp/mL), median CD4 was 558/mm. No specific adverse events related to BLV were reported and no HIV drug modification was needed. The response rates by per protocol and intent-to-treat analysis are shown in figure 3 and 4.
Figure 3: Biochemical and virologic response per protocol analysis
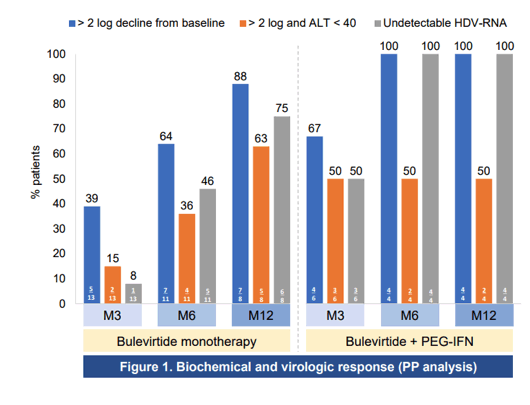
Figure 4: Biochemical and virologic response intent-to-treat analysis
Conclusion: In this first real-world cohort of HIV/HBV/HDV patients, daily administration of BLV 2 mg for 12 months was safe and well tolerated with no impact on CD4, HIV viral suppression or HIV treatment regimen. Strong HDV antiviral and biochemical responses were observed in real-life irrespective of whether PEG-IFN was added to BLV or not. Of note, biochemical and virological responses gradually increased over time in patients treated by bulevirtide monotherapy.
The Case-based Liver Workshop which takes place as a pre-meeting prior to the big CROI opening was full of very interesting case presentations this year. At this year workshop one of the cases was on epidemiology, clinical course and management issues of hepatitis delta in people living with HIV (17). Hepatitis delta virus is a small defective enveloped RNA Virus, which requires the coinfection with HBV to complete its life cycle. Therefore, hepatitis delta screening is now recommended by several guidelines (EASL) in all individuals who live with chronic hepatitis B. In the hepatitis delta lecture the concept of reflex delta screening in individuals with a positive HBs-Ag was presented (18). In this study the impact of an HDV screening program by reflex anti-HDV testing in all HBsAg-positive samples was evaluated and compared to the results before and after its implementation (see figure 5).
Figure 5: Anti-HDV reflex testing in all HBsAg+ samples (Palom et al. JHEP Rep 2022) .
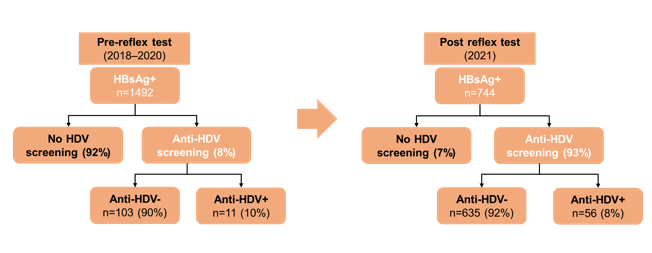
In the period before reflex testing, the percentage of delta testing was 8%, after implementing automated testing it went up to 93% which led to a 5-fold increase in the absolute number of HDV infections.
In summary, HDV is underdiagnosed despite a high prevalence in some countries and selected populations. Therefore, it is recommended to screen all HBsAg-positive individuals independently of risk factors.
Hepatitis C
At this year CROI, results from the TARGET3D study were presented for the first time. TARFET3D evaluated the efficacy and safety of glecaprevir-pibrentasvir for four weeks among people with recent HCV (see figure 6).
Figure 6: Study design TARGET3D
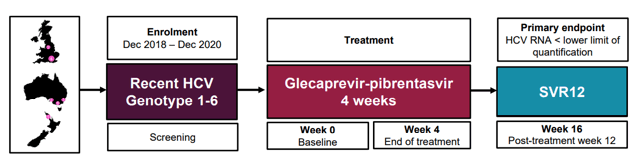
Recent primary HCV infection was defined as first positive anti-HCV Ab +/- HCV RNA within 6 months of enrolment and one of the following: 1. HCV seroconversion within 18 months, 2. Acute clinical hepatitis within 12 months (jaundice or ALT >10x ULN) or 3. Acute asymptomatic hepatitis within 12 months (ALT >5x ULN). Recent HCV reinfection was defined as new HCV RNA within 6 months of enrolment, with prior documented spontaneous or treatment-induced HCV clearance. Overall, 23 patients were included. Primary endpoint was SVR12. Results of the primary endpoint are shown in figure 7.
Figure 7: Results of the primary endpoint by ITT and PP-analysis.
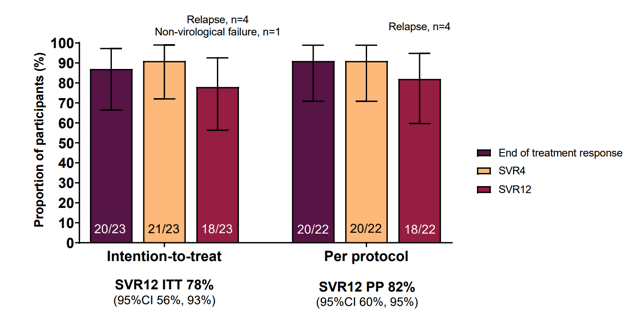
Overall, SVR rates were high with 82% in the per protocol analysis but somewhat lower than reported for other treatment regimens. This was mainly due to the occurrence of 4 virological relapses. The characteristics of the 4 relapse patients are summarized in Table 3.
Table 4: Results: Participants with virological failure
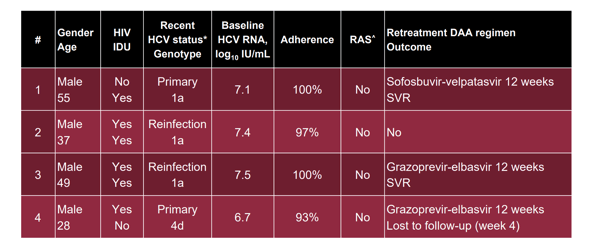
Interestingly, virological failures were only noted in individuals with a baseline viral load higher than 6log10 IU/mL. Longer DAA retreatment led to successful SVR in all subjects where post relapse information was available.
Despite achieving sustained viral response (SVR) with direct-acting antivirals (DAA), some individuals with HCV chronic infection remain at risk of developing liver complications. An interesting poster presentation investigated the incidence and underlying risk factors for long-term liver events in patients with chronic HCV infection after SVR (11).
HCV-mono-infected and HIV/HCV-coinfected individuals from the GEHEP-011 cohort with a liver stiffness prior to treatment of ≥9.5 kPa who achieved SVR with DAA-based therapy and also had a LS measurement at SVR were included into this study. Main outcome was emergence of a liver disease complication after SVR. Overall, 1116 participants were followed for a median of 58 (42 - 69) months. Overall, 677 study participants had a concomitant HIV infection (61%) and 675 had already cirrhosis prior to DAA therapy (61%).
Figure 8 summarizes the main clinical findings.
Figure 8: Appearance of liver complications
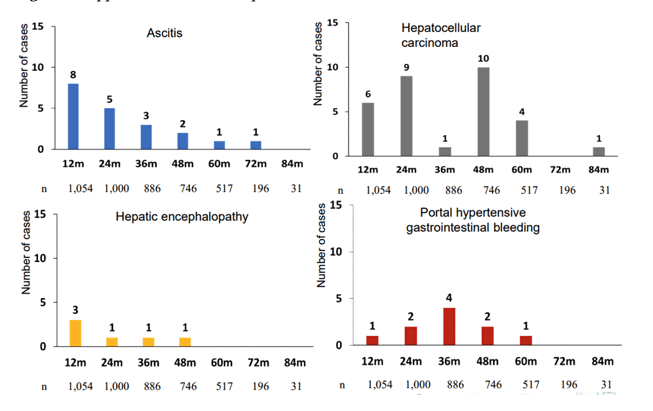
These findings demonstrate that liver complications continue to occur after long-term SVR, although the overall incidence is low. HCC clearly represents the most frequent liver disease complication observed.
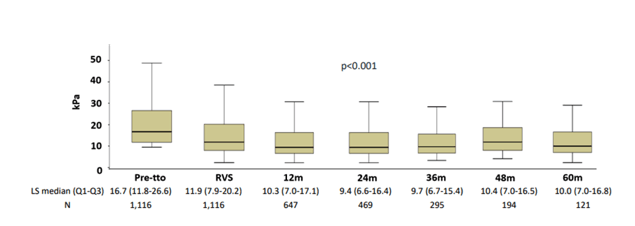
Another study looked at long-term changes in liver stiffness after SVR in patients with chronic HCV infection and advanced fibrosis (12). HCV-mono-infected and HIV/HCV-coinfected individuals from the GEHEP-011 cohort were included if their LS prior to DAA treatment was ≥9.5 kPa and if they achieved SVR with DAA-based therapy. Also study subjects needed to have a LS measurement at SVR available. LS was evaluated pre-treatment, at RVS and then annually until 60 months. The following LS categories were considered: ≤7.2 kPa; 7.3 to 9.4 kPa; 9.5 to 13.9 kPa; 14 to 20.9 kPa; and ≥ 21 kPa. LS normalization: attaining LS values ≤ 7.2 kPa after SVR. Overall, 1116 individuals were included with 61% being HIV-coinfected and 61% having cirrhosis at baseline, respectively. Liver stiffness evolution over follow-up time for the study population is shown in figure 9.
Figure 9: LS evolution during follow-up.
The liver stiffness evolution before treatment and at the last available measurement is shown in figure 10.
Figure 10: Liver stiffness evolution before treatment and at the last available measurement.
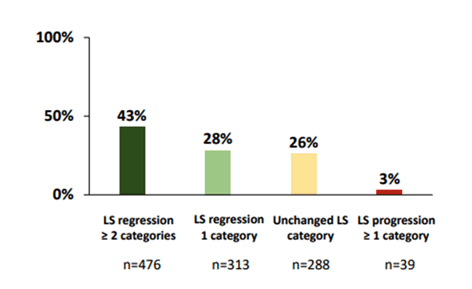
Moreover, after SVR, LS decreases significantly. This reduction is quantitatively greater at the time of SVR and the following year. In a large majority of patients, this improvement also leads to a shift to lower LS categories. Specifically, more than a quarter of individuals achieved LS normalization during the follow-up., in one third of patients, LS does not change or even increases. LS normalization according to HIV-coinfection did not differ from HCV mono-infected subjects (27% versus 31%; p=0.052). Further identification of factors associated with LS progression after SVR and specifically HIV coinfection impact, could be useful to select patients on whom screening measures for liver events after cure should be focused.
Another interesting topic in the poster session was PK levels of DAAs during pregnancy (13). Treatment of hepatitis C virus (HCV) during pregnancy with direct-acting antivirals indeed could have several benefits. First of all, it could help to decrease perinatal HCV transmission. In addition, curative HCV treatment could be provided during a time of high healthcare engagement and improved health insurance provision and thereby reduce the morbidity associated with challenges in postpartum HCV treatment and HCV testing of perinatally exposed children. Due to the physiologic changes in pregnancy, pharmacokinetic (PK) profiles of medications during pregnancy is a critical step. A PK study ledipasvir/sofosbuvir in 9 pregnant women showed similar ledipasvir and sofosbuvir concentrations but lower GS-331007 concentrations (the inactive metabolite of sofosbuvir) with 100% sustained viral response (SVR) and no safety concerns (19). However, nowadays a pan-genotypic HCV treatment regimen is recommended. The objective of the current study was therefore to compare the PK parameters of sofosbuvir/velpatasvir (SOF/VEL) in pregnant versus non-pregnant women, and to assess delivery outcomes and viral response (13). In this open-label phase I study 10 pregnant mothers were included which completed study medication and delivery visit. 2 were subsequently lost to follow-up. The pk-parameters of pregnant women were compared with pk parameters from 25 non-pregnant women. 73% (n=8) of the pregnant women had HCV genotype 1 infection, the rest genotype 3 infection. The main PK results are summarized in table 5.
Table 5: PK parameters for SOF, GS-331007 and VEL for both study groups.
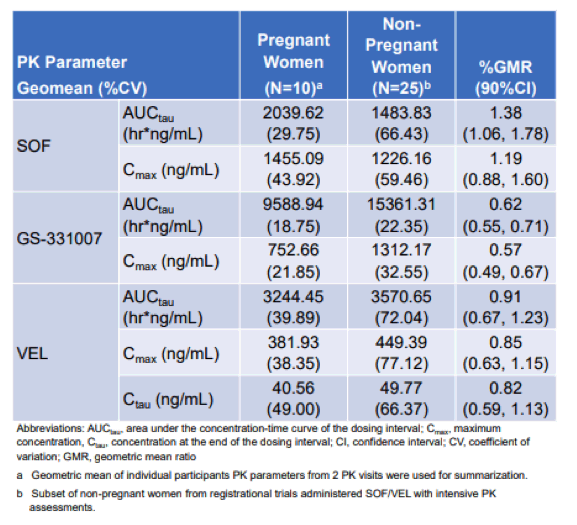
In pregnancy, VEL exposures and SOF maximum concentration (Cmax) were similar to historic data, but SOF area under the curve (AUC) was 38% higher and GS-331007 AUC and Cmax were 38% and 43% lower, respectively. This could be due to slowed gastrointestinal motility and increased renal clearance in pregnancy. A 100 % cure rate was observed for all maternal participants that completed their SVR visit (n=8). No detectable HCV RNA was found among any infant tested (n=8). No adverse safety concerns were noted.
Another interesting poster presentation evaluated the impact of DAA therapy and achieving SVR on neurocognitive function (14). HIV and Hepatitis C co-infection is known to be associated with impaired immune recovery and cognitive impairment. Clearly, the introduction of direct-acting antivirals (DAA) has dramatically changed the management of HCV due to its efficacy and favorable safety profile. In the study presented laboratory, clinical, and neuropsychiatric parameters before and after DAA therapy and HCV eradication were studied in PWH on stable antiretroviral therapy (14). Study participants were included who initiated ART immediately during acute HIV infection (AHI), were on at least 24 weeks of ART between May 2009 and July 2022, had laboratory-confirmed Hepatitis C and completed DAA therapy with sustained virologic response. HCV seroconversion after ≥24 weeks of ART between May 2009 - Jul 2022 was noted in 79 individuals. 50 of them completed DAA therapy and achieved SVR. Overall, 100% of study subjects were males, median age: 30 years (IQR: 26-35), AHI to HCV seroconversion: 192 weeks (IQR: 96-312) and median time from HCV seroconversion to DAA initiation: 58 weeks (IQR: 27-70). The study assessed the following cognitive parameters: Fine motor speed and dexterity (by using the Grooved Pegboard test (GPB)), Psychomotor speed (with Color Trails 1 (CT1) and Trails Making A (TMA)), Executive functioning (with Color Trails 2 (CT2)) and NPZ4 as an average of z-score for each test.
After treatment with DAA and achieving SVR levels of liver transaminases (AST & ALT) declined (p<0.001) and total cholesterol, low-density lipoprotein (LDL-C), and triglycerides increased (p<0.01). CD4/CD8 ratio increased (0.91 vs. 0.97, p=0.012) but CD4+ and CD8+ T cell counts did not significantly change. Stress scores measured by DT increased (1.7 vs. 2.7,p=0.045) whereas PHQ-9 scores, frequency of moderate and moderate-to-severe depression as well as frequency of peripheral neuropathy remained unchanged. NPZ-4 (p=0.004) and z-Trails Making A (p=0.028) improved significantly see figure 11). Interestingly, compared to other subjects from the acute HIV cohort with HIV mono-infection,
HIV/HCV coinfected participants showed absence of improvement in CD4/CD8 ratio and neuropsychiatric parameters before HCV seroconversion until DAA initiation over a period of 87 weeks (IQR 64,113).
Figure 11: Cognitive test z-scores before and after DAA treatment and SVR achievement.
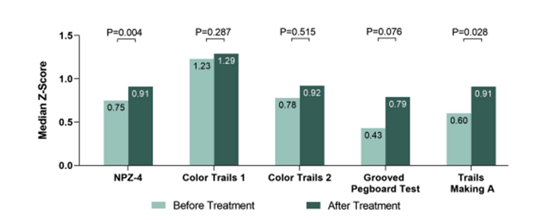
In conclusion, in a group of PWH on stable ART, an increased CD4/CD8 ratio & improved cognitive performance was observed after successful treatment of HCV with DAAs.
HIV and fatty liver disease
In the "Themed Discussion" session titled: "Steatohepatitis: Sex differences and other risk factors", four abstracts dealing with aspects of fatty liver disease in HIV were discussed (5-8). One of the studies presented assessed hepatic steatosis and fibrosis among women with HIV switching to integrase inhibitor-based therapies (INSTIs) vs non-INSTI based ART (5) The study population consisted of cisgender women co-enrolled in the SPPACE INSTI and Liver Disease Reproductive Aging (LIVRA) studies. Inclusion criteria were being on ART for at least 2 years with an HIV VL <200 cop/mL. Women who were HBSAg+, with untreated HCV, decompensated cirrhosis, or heavy alcohol use (>12 drinks/week) were excluded from this study. Study outcomes were liver steatosis (CAP) = controlled attenuation parameter (defined as significant steatosis ≥ 248 dB/m) and liver stiffness (LS) = shear wave elastography (relevant fibrosis defined as ≥7.1 kPa) and FibroScan AST (FAST) Score ≥ 0.67. Model-adjusted differences in measures of hepatic steatosis, fibrosis, and FAST scores between INSTI & non-INSTI groups are shown in figure 12. Women on INSTIs had greater LS (fibrosis) and FAST scores within 1 year of switch compared to non-INSTI Control. Model-adjusted odds of hepatic steatosis and moderate fibrosis in INSTI vs non-INSTI groups are shown in figure 13. Women on INSTIs had 3.6 greater odds of having hepatic steatosis within 1 year of switch compared to non-INSTI Controls.
Figure 12: Model-adjusted differences in measures of hepatic steatosis, fibrosis, and FAST scores between INSTI & non-INSTI groups.
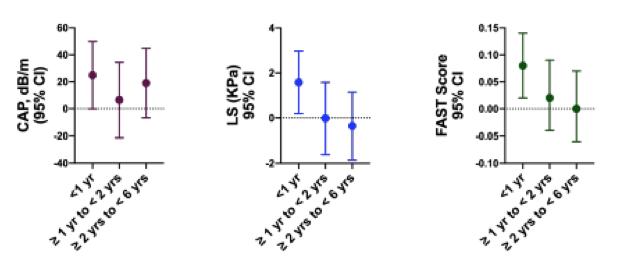
Figure 13: Model-adjusted odds of hepatic steatosis and moderate fibrosis in INSTI vs non-INSTI groups.
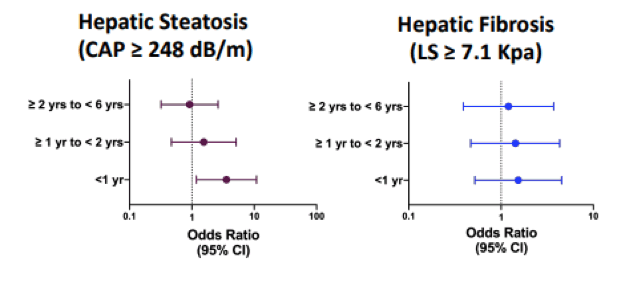
Conclusion: Women with HIV switching to INSTIs had a greater odd of having hepatic steatosis but not moderate fibrosis within the 1st year of switch compared to women on non-INSTIs. Women starting INSTIs need close monitoring of metabolic changes and low threshold to pursue non-invasive hepatic fibrosis testing.
A second study from the themed poster discussion assessed the prevalence of liver steatosis and fibrosis in PLHIV and explored associations with demographic-, metabolic-, environmental-, and HIV-specific factors, including antiretroviral therapy (ART) (6). Cross-sectional data was extracted from the 2000HIV cohort from the Netherlands. Overall, 1075 PLHIV on long-term ART were included. Steatosis (controlled attenuation parameter, CAP) and fibrosis (liver stiffness measurement, LSM) were determined by transient elastography. The results of multivariable logistic regression analysis with steatosis (left table) or fibrosis (right table) are shown in figure 14.
Figure 14: Steatosis and fibrosis findings and associated risk factors
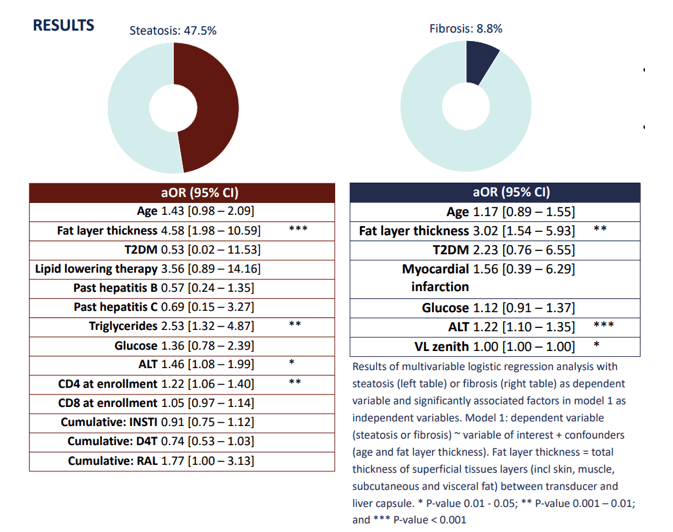
Liver steatosis and fibrosis affected nearly one in two and one in ten PLHIV in this cohort, respectively. Interestingly, NAFLD was most strongly associated with traditional NAFLD risk factors including elevated triglycerides and glucose. Of HIV-specific factors, only exposure to stavudine (D4T), integrase strand transfer inhibitors (INSTIs), and CD4+ and CD8+ T-cell counts at enrollment were associated with steatosis. CD4+ T cell count at enrollment remained predictive of steatosis in multivariable analysis. The question in how far modern integrase inhibitor therapy contributes to hepatic steatosis remains under debate.
A third study looked at potential sex differences in the occurrence of metabolic-dysfunction-associated fatty liver disease (MAFLD) in People with HIV (PWH) (7). In the general population, sex differences seem to exist in frequency and severity of MAFLD with higher prevalence of MAFLD in men but higher incidence of liver fibrosis in women. Less is known about sex differences in MAFLD and liver fibrosis in the setting of HIV infection. Therefore, this study investigated sex differences in the prevalence and severity of MAFLD in an HIV population. MAFLD was defined as: Presence of hepatic steatosis, diagnosed as CAP>270 dB/m plus any among type 2 diabetes, overweight (BMI>25 Kg/m2) or two other metabolic abnormalities. Significant liver fibrosis was diagnosed as LSM>8 kPa.
The baseline characteristics of the study population are summarized in table 6.
Table 6: Baseline characteristics
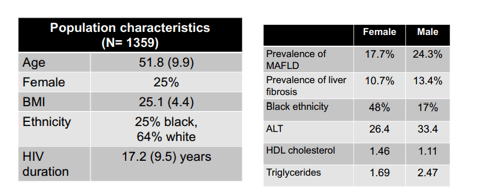
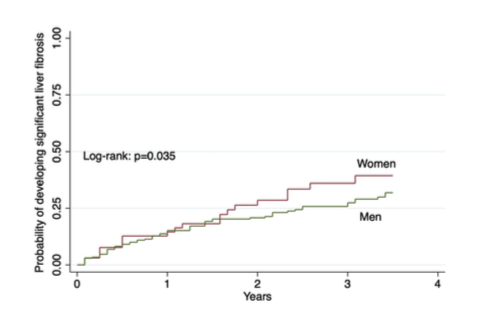
The incidence of MAFLD was similar between women and men with HIV. The incidence of liver fibrosis however, was higher in women vs. men with HIV (7.0 vs. 5.9 per 100 PY particularly after 50 years old). On multivariable cox regression and after age adjustment: MAFLD (aHR 3.3, 95% CI 2.0-5.6) and female sex (aHR 2.2, 95% CI 1.3-3.5) were independent predictors of developing significant liver fibrosis while CD4 cell count was protective (aHR 0.99, 95% CI 0.99-0.99). Figure 15 shows the development of significant liver fibrosis by biological sex category.
Figure 15: Development of significant liver fibrosis by biological sex category.
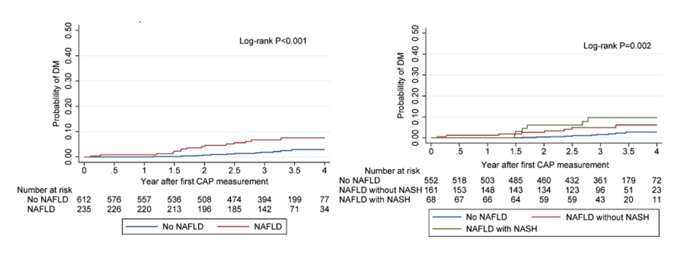
The fourth presentation in the Steatohepatitis themed poster discussion raised the question how NAFLD and its combination with NASH predicts diabetes development in people with HIV (8). Within this Thai HIV cohort study the association of non-alcoholic fatty liver disease (NAFLD) plus or minus a concurrent diagnosis of non-alcoholic steatohepatitis (NASH) with incident diabetes mellitus (DM) was assessed. PWH aged ≥18 years, on stable antiretroviral therapy (ART) from a long-term cohort in Bangkok were eligible for enrollment. Liver stiffness (LS) and controlled attenuated parameter (CAP) values were collected from FibroScan. NAFLD was defined as CAP >248 dB/m, whereas NASH with significant activity and liver fibrosis was defined as FibroScan-AST (FAST) score ≥0.67. Baseline was defined as the first FibroScan date. PWH with hepatitis B or C virus infection, those with excessive alcohol consumption or with DM diagnosed prior to baseline were excludeed. Clinical endpoint was new-onset DM defined as fasting glucose at least 126 mg/dL on two consecutive study visits, or reported onset of diabetes and initiation of diabetes treatment. Among 847 eligible PLWH, the median age at baseline was 46 (IQR 39-52) years (43% female). Median baseline CD4 was 588 (IQR 443-759) cells/mm3 and 90% had HIV-1 RNA <50 copies/mL. the median CAP value and FAST score was 215 (IQR 184-254) dB/m and 0.22 (IQR 0.12-0.43), respectively. 28% (235/847) and 15% (116/781) had NAFLD and NASH at baseline, respectively. During a median follow-up time of 3.3 (IQR 2.7-3.6) years, 28 developed DM (incidence rate=11.0 [95%CI 7.6-15.9] per 1000 person-years). Most interestingly, baseline NAFLD was associated with an increased risk of incident DM (hazard ratio[HR]: 2.8, 95%CI 1.3-6.4) after adjusting for age, sex, family history of DM, duration of ART, and didanosine exposure, and time-updated BMI, hypertension and dyslipidemia. Moreover, combined NAFLD and NASH with significant activity and liver fibrosis at baseline increased the risk of incident DM (HR: 3.1, 95%CI 1.1-9.3). Figure 16A and 16B show that the probability of incident DM was higher in PWH with NAFLD or NAFLD/NASH at baseline. Baseline NASH alone also showed a non-significant but elevated risk of incident DM (HR:1.8, 0.7-4.4).
Figure 16A and 16B: Probability of DM development by NAFLD status (A) and by NAFLD with or without NASH with fibrosis (B).
In a separate adjusted analysis including DM at baseline (but excluding NASH with fibrosis at baseline), NAFLD with DM at baseline (HR: 2.82, 95%CI 1.17-6.78) and TAF use (HR: 2.59, 95% CI: 1.12-6.02) was associated with incident NASH with significant activity and liver fibrosis. In summary, NAFLD alone or combined with NASH was strongly associated with DM development in PLWH, highlighting the need for DM risk assessment and management in PLWH with NAFLD.
In the poster session a large multi-cohort study was presented which assessed risk factors for liver fibrosis progression in PLH (15). Aim of the study was to investigate the effect of MAFLD and antiretroviral exposure on liver fibrosis progression in people living with HIV (PWH). This was a longitudinal study of three large prospective cohorts of PWH in Italy, Germany and Canada. All patients with at least two transient elastography with controlled attenuation parameters (CAP) exams were included. Liver fibrosis progression was defined as development of significant LF (defined as liver stiffness >8 kPa), or transition to cirrhosis (defined as liver stiffness >13 kPa for those with liver stiffness >8 but < 13 kPa at baseline). MAFLD was defined according to Eslam criteria: presence of hepatic steatosis (CAP>248 dB/m), plus any among type 2 diabetes, overweight (BMI>25 Kg/m2) or two other metabolic abnormalities. Interestingly, liver fibrosis progression occurred in a significant proportion of PWH. Metabolic associated fatty liver disease, BMI and weight gain were associated with a higher risk of liver fibrosis progression. Overall, men had a lower risk of liver fibrosis regression. Virus hepatitis B and C coinfection were not associated with liver fibrosis progression, probably due to adequate management of chronic HBV infection and successful treatment of HCV. The transition of liver fibrosis over time and the association with various factors is shown in table 7.
Table 7: The transition (progression or regression) of liver fibrosis by Markov model (odds ratios with confidence intervals).
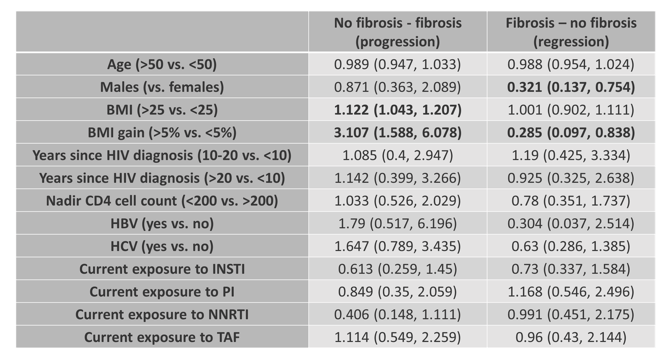
Conclusion: Liver fibrosis progression occurs in a significant proportion of PWH, higher than reported in literature for the general population. Its main drivers include metabolic health variables, while ART exposure does not seem to impact liver fibrosis progression. People living with HIV should be routinely screened for liver fibrosis, regardless of viral hepatitis co-infection.
Summary
• In adults with HIV/HBV coinfection initiating antiviral therapy for the first time, after 48 weeks, significantly more participants on B/F/TAF versus DTG + F/TDF had HBV DNA < 29 IU/mL.
• Several baseline factors were determined to be predictors of HBV DNA suppression, including B/F/TAF treatment, HBeAg-negative status, HBV DNA < 8 log10 and ALT > ULN at baseline.
• ALT > ULN and CD4 ≥ 200 cells/μL at baseline were predictors of HBeAg and HBsAg loss.
• PAGE-B is a valid score to estimate HCC risk in individuals with HIV/HBV coinfection. If the PAGE-B score is <10 this is associated with a very high negative predictive value. Indeed, the PAGE-B score may help to decide in whom HCC screening is needed.
• A decline in circulating HBV RNA and HBcrAg levels during tenofovir therapy is more likely in PLWH with functional HBV cure than in those without cure.
• In a real-world cohort of HIV/HBV/HDV patients, daily administration of bulevirtide (BLV) 2 mg for 12 months was safe and well tolerated with no impact on CD4, HIV viral suppression or HIV treatment regimen. Strong HDV antiviral and biochemical responses were observed irrespective of whether PEG-IFN was added to BLV or not.
• HDV is underdiagnosed despite a high prevalence in some countries and selected populations. Therefore, it is recommended to screen all HBsAg-positive individuals independently of risk factors.
• HCV clearance under DAA treatment with glecaprevir-pibrentasvir for four weeks was lower than seen with longer treatment durations (≥six weeks) among people with recent HCV infection. Efficacy was clearly associated with level of baseline HCV RNA.
• Liver complications continue to occur after long-term SVR, although the overall incidence is low. HCC remains the most frequent complication.
• After SVR, liver stiffness decreases significantly. This reduction is quantitatively greater at the time of SVR and the following year. In a large majority of patients, this improvement also leads to a shift to lower LS categories. Specifically, more than a quarter of individuals achieved LS normalization during the follow-up.
• SOF/VEL administration during pregnancy showed a favorable PK profile with reassuring preliminary safety and efficacy outcomes.
• An increased CD4/CD8 ratio and improved cognitive performance was observed after successful treatment of HCV with DAAs in PWH.
• Women with HIV switching to INSTIs have a greater odd of having hepatic steatosis but not moderate fibrosis within the 1st year of switch compared to women on non-INSTIs. Women starting INSTIs need close monitoring of metabolic changes and low threshold to pursue non-invasive hepatic fibrosis testing.
• NAFLD in PLHIV is most strongly associated with traditional metabolic risk factors.
• MAFLD seems to be a sexual dimorphic disease in PWH
• Despite having lower rates of MAFLD, women with HIV have higher incidence of significant liver fibrosis compared to men, especially after 50 years of age.
• NAFLD alone or combined with NASH is strongly associated with DM development in PLWH, highlighting the need for DM risk assessment and management in PLWH with NAFLD.
• Liver fibrosis progression occurs in a significant proportion of PWH. Its main drivers include metabolic health variables, while ART exposure does not seem to impact liver fibrosis progression. People living with HIV should be routinely screened for liver fibrosis, regardless of viral hepatitis co-infection.
References
1. Chloe L. Thio, Maraake Taddese, et al. cccDNA silencing and limited decay of infected cells in HIV-HBV (HBeAg-) during NUCs. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 115
2. Anchalee Avihingsanon et al. Predictors of Hepatitis B treatment response in People with HIV/HBV coinfection. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 116
3. Bernard Surial et al. PAGE-B score to estimate the hepatocellular carcinoma risk in People Living with HIV and HBV 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 117
4. Marianne Martinello, et al. TARGET3D: Glecaprevir-Pibrentasvir for 4 weeks in people with recent HCV infection. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 194
5. Cecile D. Lahiri, et al. Liver steatosis and fibrosis in women with HIV by integrase inhibitor use. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 610
6. Louise E. van Eekeren, et al. Prevalence and Factors Associated with NAFLD In People with HIV. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 609
7. Dana Kablawi et al. Sex differences in the association of HIV with metabolic-fatty liver disease. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 611
8. Win Min Han et al. NAFLD and its combination with NASH predict DM development in people with HIV. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 612
9. Lorin Begré et al. Kinetics of HBV RNA and core-related-antigen levels in PLWH with functional HBV cure. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 586
10. Victor de Lédinghen et al. Bulevirtide +/- PEG-IFN in HIV/HBV/HDV Co-infected Patients in Real-Life Settings. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 589
11. J. Martin Carmona et al. Long-term liver events in patients with HCV chronic infection after SVR. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 605
12. Anaïs Corma-Gómez et al. Long-term changes in liver stiffness after SVR in patients with HCV chronic infection and advanced fibrosis. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 607
13. Catherine A. Chappell et al. Sofosbuvir/Velpatasvir Pharmacokinetics in Pregnant Women with Hepatitis C Virus. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 597
14. Ferron F. Ocampo et al. Clinical and laboratory outcomes of hepatitis C treatment in an acute HIV cohort. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 490
15. Giovanni Guaraldi, et al., Risk factors for liver fibrosis progression in HIV: a multi-center longitudinal study. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, abstract 617
16. Anchalee Avihingsanon et al. Week 48 results of a phase 3 randomized controlled trial of bictegravir/ emtricitabine/tenofovir alafenamide (B/F/TAF) vs dolutegravir + emtricitabine/ tenofovir disoproxil fumarate (DTG+F/TDF) as initial treatment in HIV/HBV-coinfected adults (ALLIANCE). AIDS 2022, Montreal, July 29 - 3 August, 2022. Abstract 12565
17. Charles Béguelin. Hepatitis Delta infection among persons living with HIV. 30th Conference on Retroviruses and Opportunistic Infections, Seattle, February 19-22, 2023, Case-based Liver Workshop, abstract 8
18. Palom A, et al. Implementation of anti-HDV reflex testing among HBsAg-positive individuals increases testing for hepatitis D. JHEP Rep. 2022 Jul 21;4(10):100547.
19. Chappell CA, et al. Ledipasvir plus sofosbuvir in pregnant women with hepatitis C virus infection: a phase 1 pharmacokinetic study. Lancet Microbe. 2020;1:e200-e208.
|
| |
|
 |
 |
|
|