 |
 |
 |
| |
Weight and Metabolic Benefits With TDF/FTC/DTG After 4 Years of TAF/FTC+DTG
|
| |
| |
30th CROI, Conference on Retroviruses and Opportunistic Infections, February 19-22, 2023, Seattle
Mark Mascolini
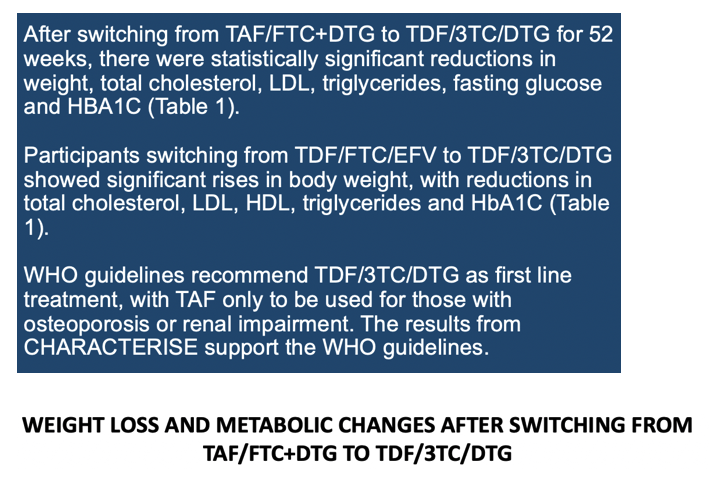
South Africans taking tenofovir alafenamide/emtricitabine plus dolutegravir (TAF/FTC+DTG) for 192 weeks gained significantly more weight than people taking tenofovir disoproxil fumarate (TDF)/FTC+DTG) in the ADVANCE trial [1]. But 52 weeks after people switched from the TAF combination to the TDF combination, CHARACTERISE study investigators charted significant improvements in weight, body mass index (BMI), total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, fasting glucose, and HbA1c (like glucose, a diabetes metric) [2]. The researchers believe their findings support WHO guidelines to start antiretroviral therapy with TDF/3TC/DTG and to substitute TAF for TDF only in people with osteoporosis or impaired kidney function.
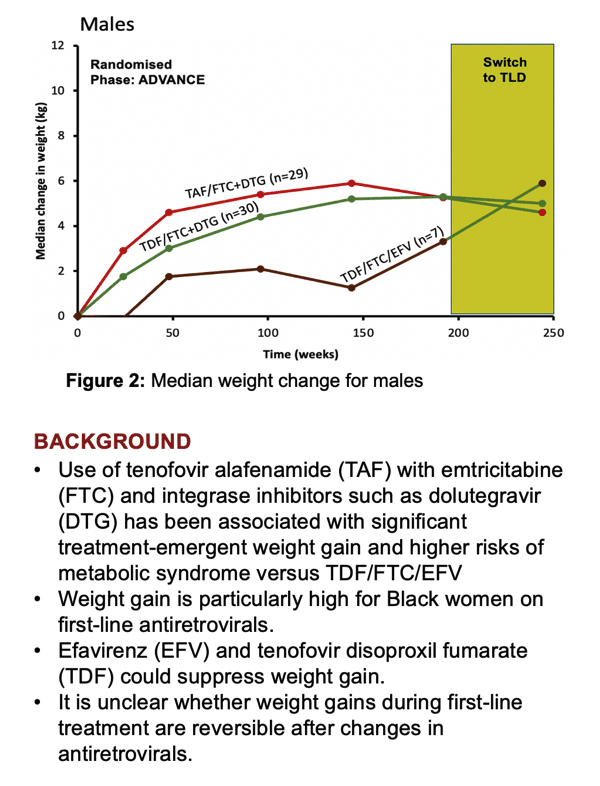
Much work has linked regimens containing TAF and an integrase inhibitor like DTG to weight gains and metabolic turmoil, especially in black women taking these antiretrovirals as first-line therapy, CHARACTERISE investigators noted. TDF has long been tied to suppression of weight gain-as well as to kidney and bone toxicity. But whether substituting TDF for TAF after years of a TAF regimen would reverse TAF-associated weight gain remained unclear.
Conclusion of the ADVANCE trial offered the perfect opportunity to find out. ADVANCE randomized 1053 antiretroviral-naive South Africans to TAF/FTC+DTG, TDF/FTC+DTG, or TDF/FTC/efavirenz (EFV) for 192 weeks. After 48 weeks the two DTG regimens proved virologically noninferior to the EFV regimen, the local standard-care regimen at the time [1]. People taking a DTG combination-especially those also taking TAF-gained more weight than the EFV group. The TAF combination affected kidneys and bone less than the two TDF regimens.
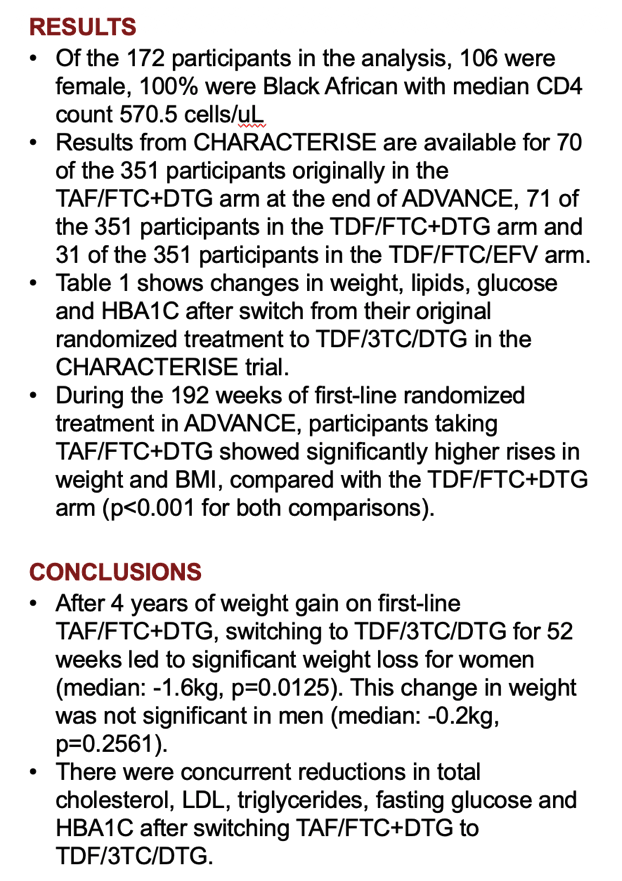
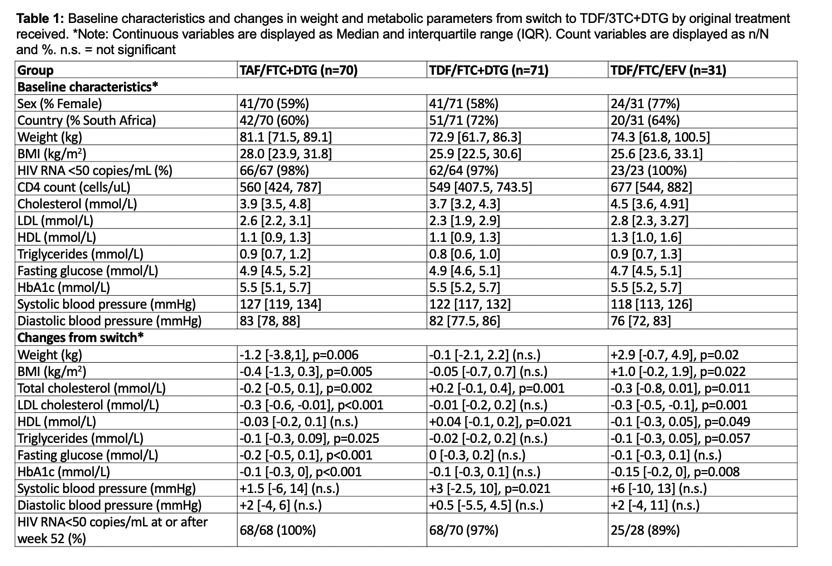
Among the 172 people who completed 192 weeks on their originally assigned regimen then took TDF/FTC/DTG for 52 weeks, 70 came from the original TAF/FTC+DTG group, 71 from the TDF/FTC+DTG group, and 31 from the TDF/FTC/EFV group. At the 192-week switch, the two original DTG groups had lower proportions of women (59% and 58%) than the original EFV group (77%). Weight and body mass index (BMI) were at the 192-week switch were higher in the original TAF group than in the other two groups (median BMI 28.0 vs 25.9 vs 25.6 kg/m3). Across the three groups, 97% or more had a viral load below 50 copies at week 192, and median CD4 count measured 570.5 for the three combined groups. Everyone was black African.
During the 52 weeks in which everyone took TDF/FTC/DTG, women (but not men) originally assigned to TAF/FTC+DTG had the greatest median weight loss (-1.6 kg, P = 0.0125). When the researchers combined women and men, people trading the original TAF regimen for the TDF regimen for 52 weeks had statistically significant drops in weight (-1.2 kg, P = 0.006), BMI (-0.4 kg/m2, P = 0.005), total cholesterol (-0.2 mmol/L, P = 0.002), "bad" LDL cholesterol (-0.3 mmol/L, P < 0.001), triglycerides (-0.1 mmol/L, P = 0.025), fasting glucose (-0.2 mmol/L, P < 0.001), and HbA1c (-0.1 mmol/L, P < 0.001).
Through the 52 weeks after the switch to TDF/FTC/DTG, the group who originally took that regimen had significant gains in total cholesterol (+0.2 mmol/L, P = 0.001), "good" HDL cholesterol (+0.4 mmol/L, P = 0.021), and systolic blood pressure (+3 mm Hg, P = 0.021). In the 52 weeks taking TDF/FTC/DTG, the group originally taking TDF/FTC/EFV had significant jumps in weight (+2.9 kg, P = 0.02), and BMI (+1.0 kg/m2, P = 0.022), and significant drops in total cholesterol (-0.3 mmol/L, P = 0.011), LDL cholesterol (-0.3 mmol/L, P = 0.001), HDL cholesterol (-0.1 mmol/L, P = 0.049), and HbA1c (-0.15 mmol/L, P = 0.008).
References
1. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381:803-815. DOI: 10.1056/NEJMoa1902824. https://www.nejm.org/doi/full/10.1056/NEJMoa1902824
2. Bosch BE, Akpomiemie G, Chandiwana N, et al. Weight loss and metabolic changes after switching from TAF/FTC/DTG to TDF/3TC/DTG. 30th CROI, Conference on Retroviruses and Opportunistic Infections, February 19-22, 2023, Seattle. Abstract 671.
Bronwyn Bosch1, Godspower Akpomiemie1, Nomathemba Chandiwana1, Simiso Sokhela1, Andrew Hill2, Kaitlyn McCann3, Ambar Qavi3, Manya Mirchandani3, Francois Venter1
1Ezintsha, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, 2Department of Pharmacology and Therapeutics, University of Liverpool, United Kingdom. 3Faculty of Medicine, Imperial College, London, UK

|
| |
|
 |
 |
|
|