 |
 |
 |
| |
Doravirine Does Not Drive up ASCVD Risk Through 4 Years
|
| |
| |
EACS 2023, October 18-21, 2023, Warsaw
Mark Mascolini
Treatment of HIV infection with the nonnucleoside doravirine for 4 years did not significantly change cardiovascular disease (CVD) risk as measured by the atherosclerotic CVD (ASCVD) risk score, according to analysis of two phase 3 trials of doravirine in previously untreated adults. Scores rose slightly in men, particularly black men, in these trials, but increasing age accounted for much of that upswing.
US, German, and French researchers who ran this post hoc analysis of pooled data from the DRIVE-FORWARD [2] and DRIVE-AHEAD [3] doravirine trials noted that ASCVD risk runs 1.5- to 2-fold higher in people with than without HIV infection. Because doravirine had a minimal impact on weight and did not induce abnormal lipids in phase 3 trials, the three-country team assessed ASCVD risk in doravirine-treated people in DRIVE-FORWARD and DRIVE-AHEAD. (Post hoc means the analysis was not part of the original trial plan.)
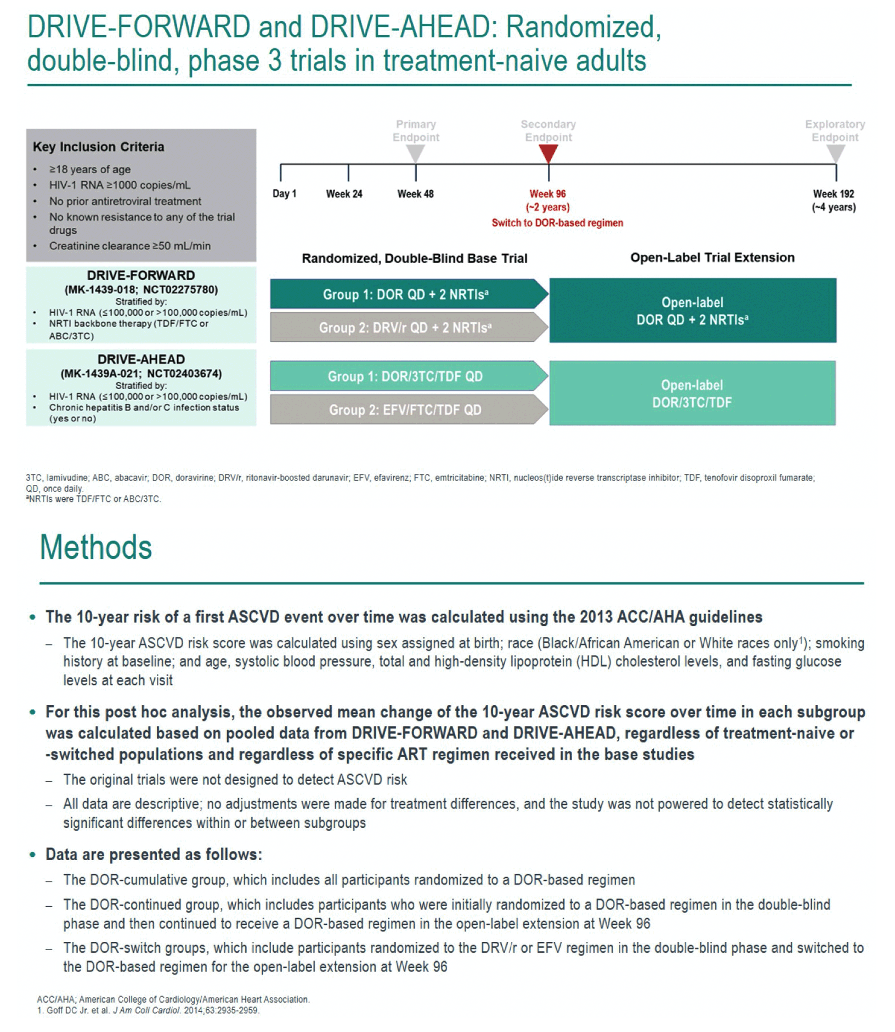
The DRIVE trials recruited adults without antiretroviral experience and no known resistance to any study drug. Researchers randomized them to doravirine plus two nucleosides/nucleotides (NRTIs) or to darunavir/ritonavir plus two NRTIs in DRIVE-FORWARD and to doravirine or efavirenz plus two NRTIs in DRIVE-AHEAD. At study week 96, people in the comparison groups could switch to a doravirine combination and everyone continued treatment to week 192.
The researchers used ACC/AHA guidelines to calculate 10-year risk of a first ASCVD event in doravirine-treated people regardless of whether they took doravirine for all 192 weeks or a comparative regimen for the first 96 weeks and regardless of what NRTIs they took. The investigators stressed that the original DRIVE trials were not planned to assess ASCVD risk and that their analysis made no adjustments for treatment differences and did not have statistical power to detect significant differences between or within subgroups.
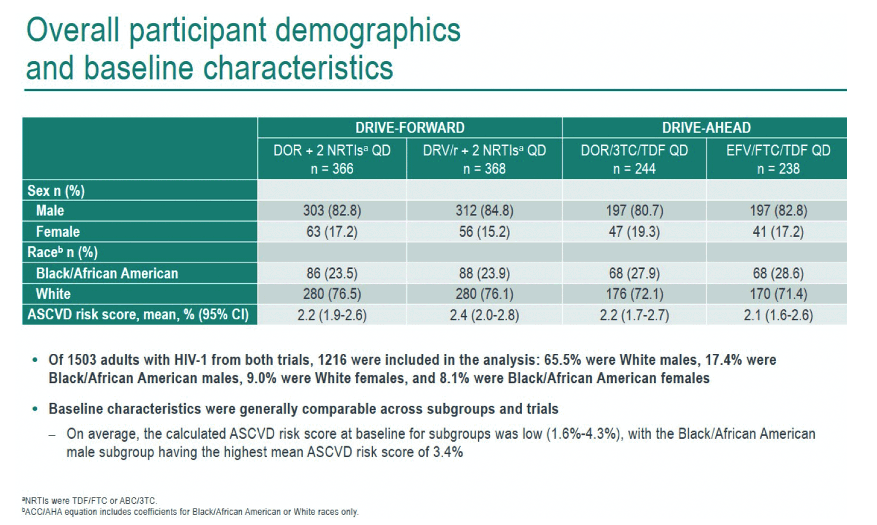
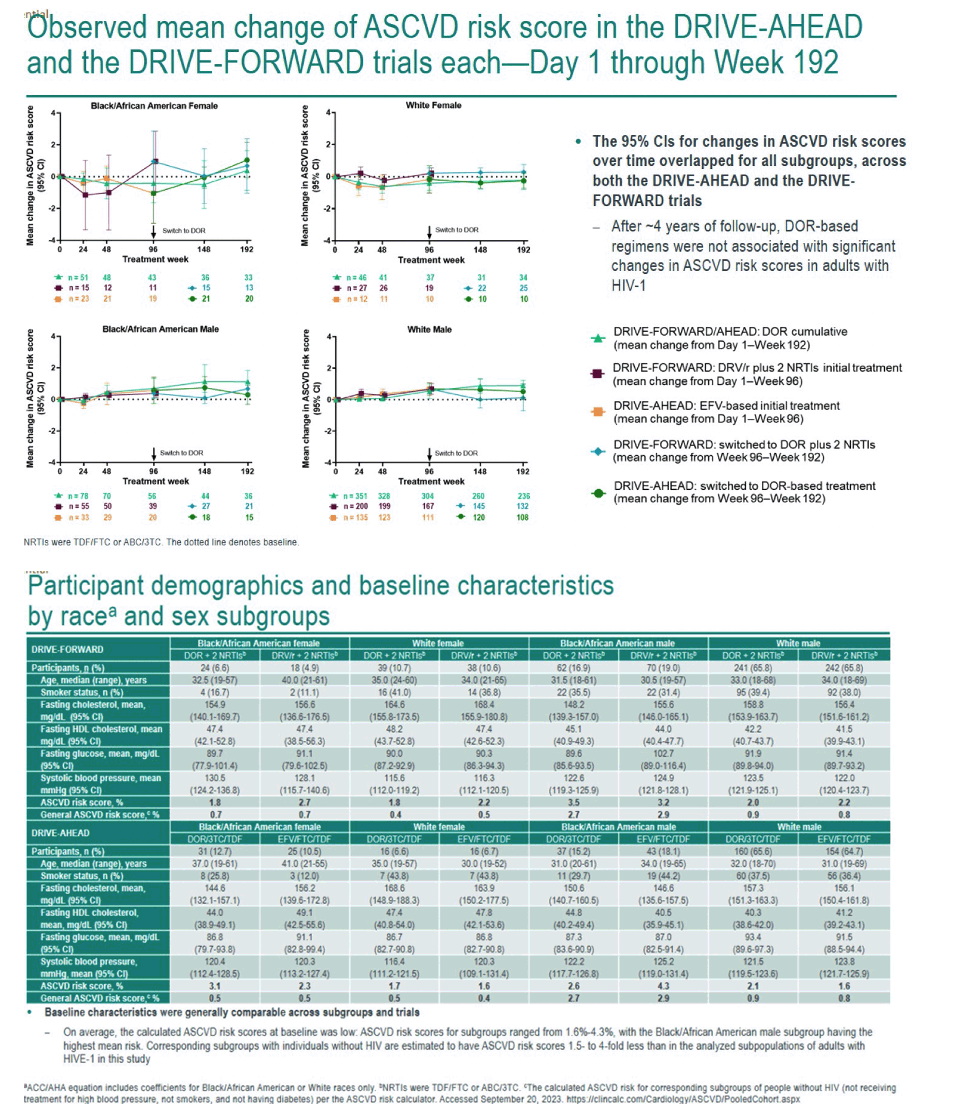
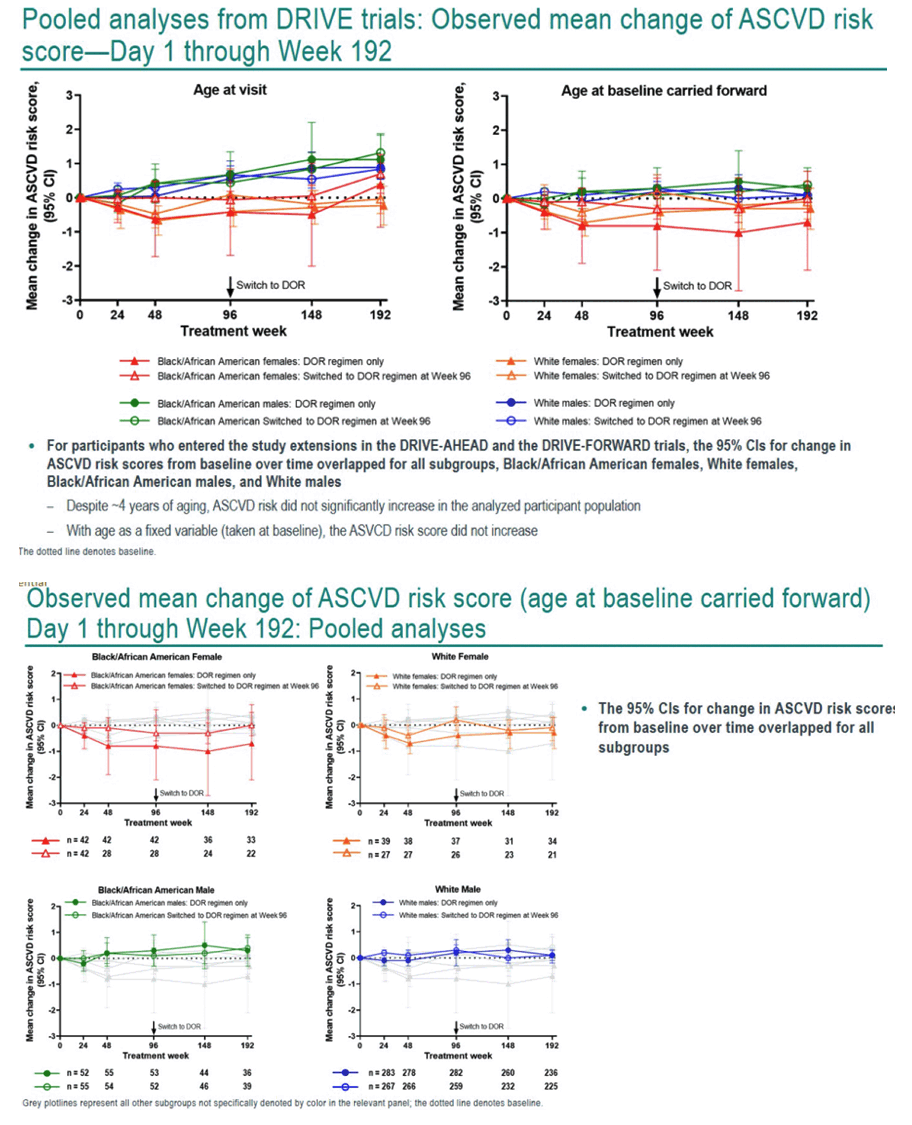
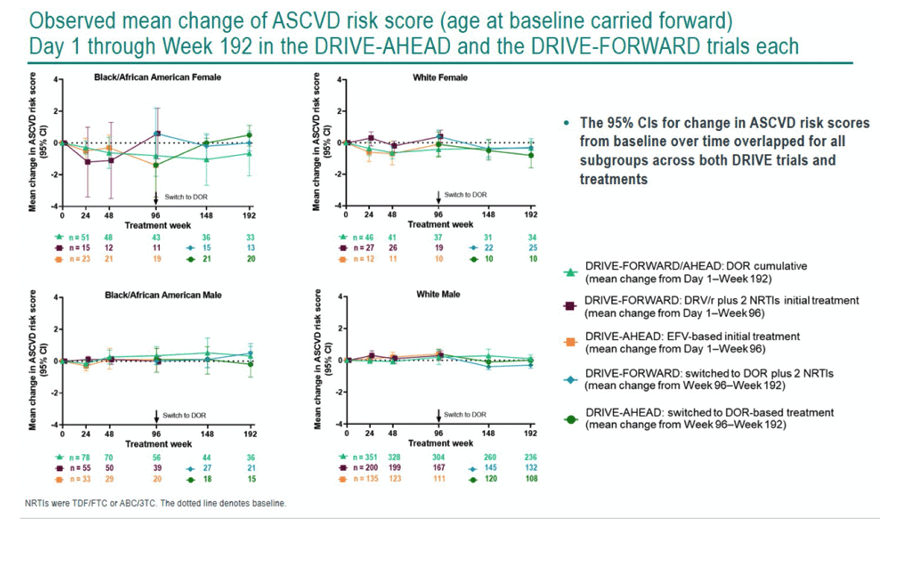
This analysis included 1216 adults from the combined DRIVE trials, 65.5% of them white men, 17.4% black men, 9.0% white women, and 8.1% black women. Baseline traits were generally similar across subgroups. When study treatment began, ASCVD risk was low, ranging from 1.6% to 4.3%, with black men running the highest risk, 3.4%.
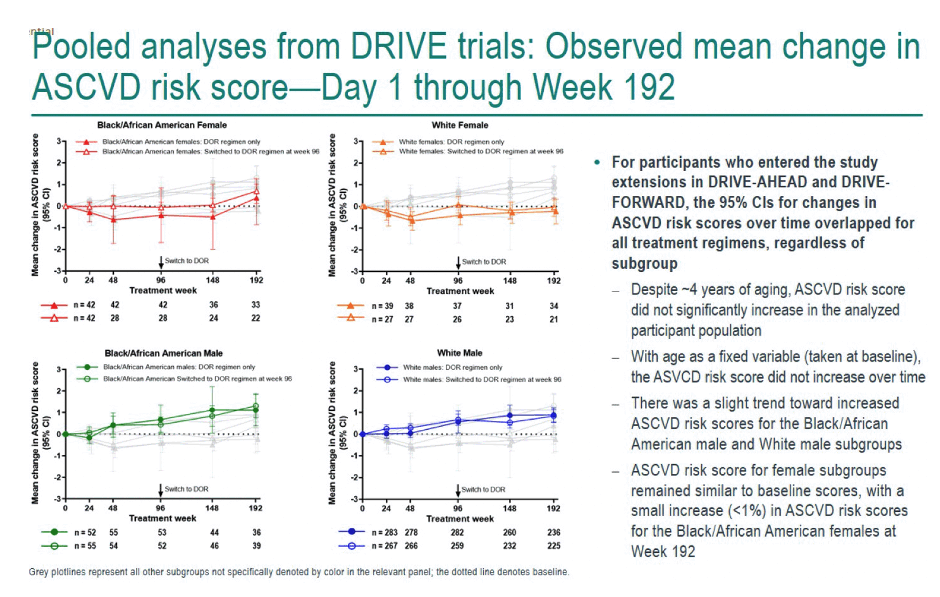
If statisticians made age at first treatment a fixed variable, ASCVD risk score did not rise significantly over 192 weeks of study. The researchers reported "a slight trend" toward higher ASCVD risk in black men and in white men. Risk scores hardly changed over time in women; at week 192 black women had less than a 1% increase in risk score. Aging in the study group was the main driver of the small climb in ASCVD risk recorded. Among trial participants who entered the doravirine extension phase at week 96, the 95% confidence intervals for change in ASCVD risk score overlapped for all treatment groups.
Because people with HIV run a higher risk for ASCVD than the general population, the DRIVE trial investigators urged that choice of antiretrovirals should not add to that risk. They proposed their findings suggest "doravirine might represent a therapeutic strategy that does not increase the risk of ASCVD for adults with HIV-1."
The researchers noted that the small number of women analyzed means firm conclusions can be drawn about ASCVD risk only after further research in women. One doravirine trial now in the works (but not yet recruiting) should help fill that gap by randomizing 600 adults, 60% of them women [4].
References
1. Hsue P, Behrens G, Xu ZJ, et al. Cardiovascular risk assessment after continuing or switching to a doravirine-based regimen using the atherosclerotic cardiovascular disease (ASCVD) risk score model. EACS 2023, October 18-21, 2023, Warsaw. Abstract OS2.03.
2. Molina JM, Squires K, Sax PE, et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 96-week results of a randomised, double-blind, non-inferiority, phase 3 trial. Lancet HIV. 2020;7:e16-e26. doi: 10.1016/S2352-3018(19)30336-4. https://pubmed.ncbi.nlm.nih.gov/31740348/
3. Orkin C, Squires KE, Molina JM, et al. Doravirine/lamivudine/tenofovir disoproxil fumarate (TDF) versus efavirenz/emtricitabine/TDF in treatment-naive adults with human immunodeficiency virus type 1 infection: week 96 results of the randomized, double-blind, phase 3 DRIVE-AHEAD noninferiority trial. Clin Infect Dis. 2021;73:33-42. doi: 10.1093/cid/ciaa822. https://academic.oup.com/cid/article/73/1/33/6041522
4. ClinicalTrials.gov. A randomised, phase 3 non-inferiority study of DOR/3TC/TDF compared to DTG/TAF/FTC in participants infected with HIV-1 starting first-line antiretroviral therapy. ClinicalTrials.gov ID NCT05924438. https://clinicaltrials.gov/study/NCT05924438

|
| |
|
 |
 |
|
|