 |
 |
 |
| |
RBD1016, an siRNA, Lowers HBsAg for
24 Weeks in Phase 1 Placebo Trial
|
| |
| |
EASL Congress 2023, June 21-24, Vienna
Mark Mascolini
RBD1016, a short interfering RNA (siRNA) agent, lowered hepatitis B surface antigen (HBsAg, a signal of active HBV infection) in a 2-group 24-week trial of people with chronic hepatitis B virus (HBV) infection [1]. The impact of RBD1016 occurred in the first days of treatment and proved greater at higher doses.
siRNA drugs are synthetic double strands of RNA designed to home to and degrade specific messenger RNAs (mRNA) of target genes. Four siRNA drugs are licensed by the FDA for rare metabolic disorders as well as for lowering lofty low-density lipoprotein cholesterol in people with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease [2].
Researchers at University of Hong Kong hospitals are conducting this ongoing double-blind, placebo-controlled phase 1 trial of RBD1016 in adults with chronic hepatitis B infection [1,3]. Participants could be naive to HBV therapy or previously treated but without hepatic fibrosis or cirrhosis. Everyone got RBD1016 or placebo plus a nucleos(t)ide analog.
The investigators split participants into a single-dose group and a multiple-dose group. Single-dose participants got either RBD1016 or placebo in a 5-to-1 ratio at rising doses of 0.3, 1, 3, and 6 mg/kg. Multiple-dose participants received 2 doses of RBD1016 or placebo on days 1 and 29 in a 6-to-2 drug-to-placebo ratio at rising doses of 3 and 6 mg/kg. At EASL the Hong Kong team showed data up to 24 weeks for doses of 0.3, 1, and 3 mg/kg in the single-dose group and 3 mg/kg in the multiple-dose group.
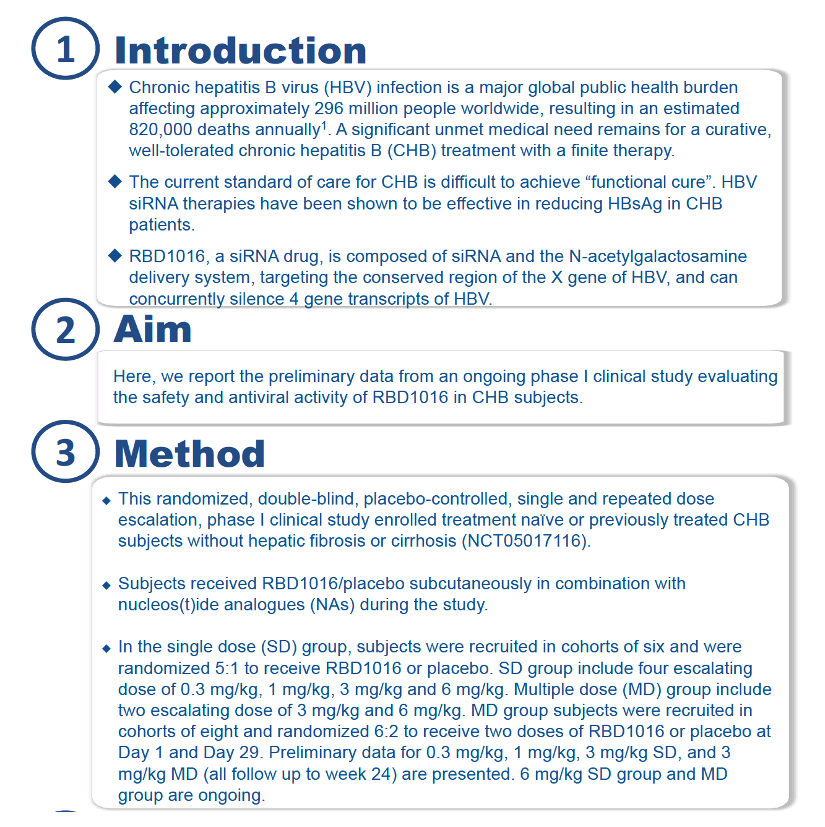
The 24 people in the single-dose group and the 16 in the multiple-dose group averaged 43.8 years in age (range 26 to 55). Overall, 55% were women and 45% men, and everyone was Chinese. Only 2 of 40 participants-1 in the single-dose group and 1 in the multiple-dose group-had never taken medications for HBV infection. HBsAg levels averaged 3358.8 IU/mL across groups but were not reported for the 6-mg/kg single- and multiple-dose groups.
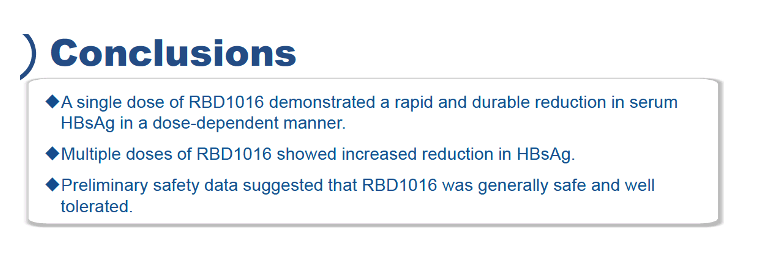
Maximum drop in serum HBsAg averaged 0.48 log10 IU/mL at week 12 in the 0.3-mg/kg single-dose group, 0.75 log10 IU/mL at week 12 in the 1-mg/kg single-dose group, 0.97 log10 IU/mL at week 16 in the 3-mg/kg single-dose group, 1.26 log10 IU/mL at week 12 in the 3-mg/kg multidose group, and 0.00 log10 IU/mL in the placebo group. These drops in HBsAg held steady through week 24. All participants who got 1 or 3 mg/kg of RBD1016 had at least a half-log maximum fall in HBsAg.
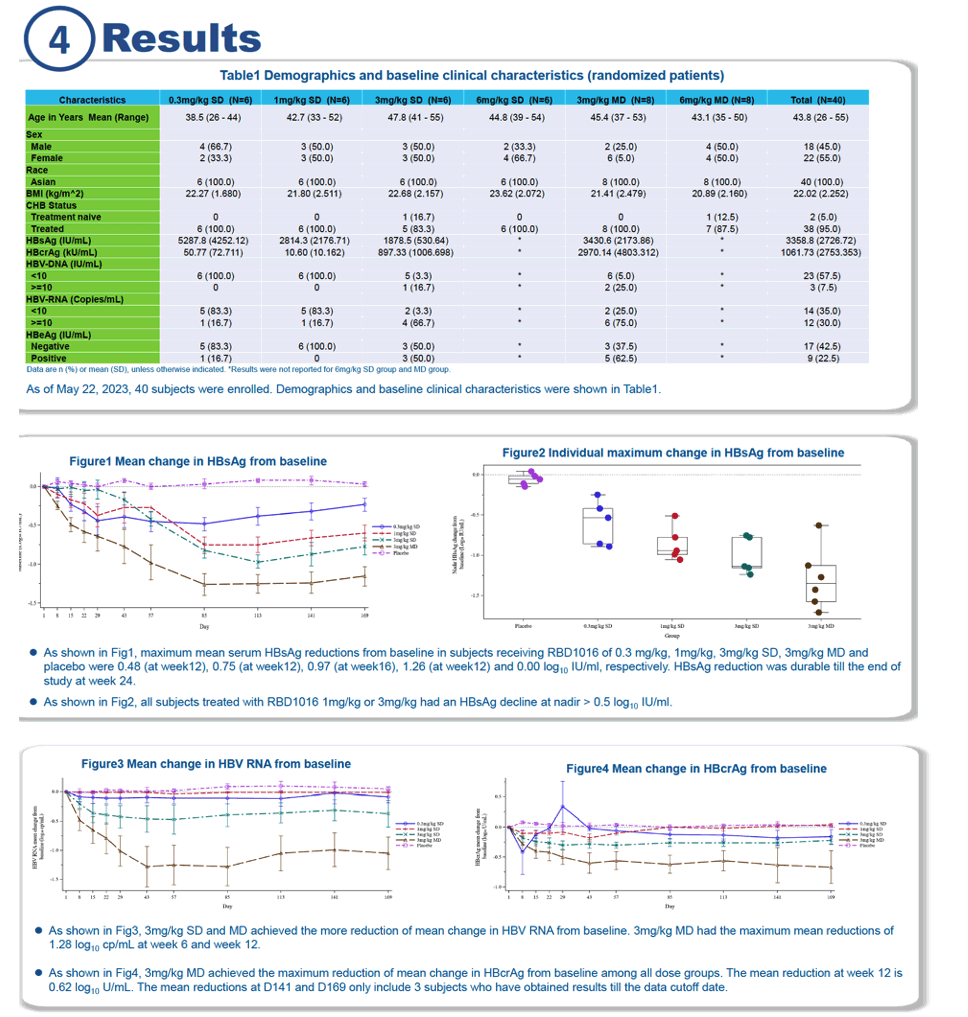
Average declines in HBV RNA proved greatest in the 3-mg/kg single- and multiple-dose group. People receiving 3 mg/kg in the multidose group had the highest average drop in HBV RNA, 1.28 log10 copies/mL, at weeks 6 and 12. The biggest average decline in hepatitis B core-related antigen (HBcrAg)-proposed as a marker for HBV disease monitoring because it reflects covalently closed circular DNA (cccDNA) [4]-came in the 3-mg/kg multidose group (0.62 log10 IU/mL at week 12).
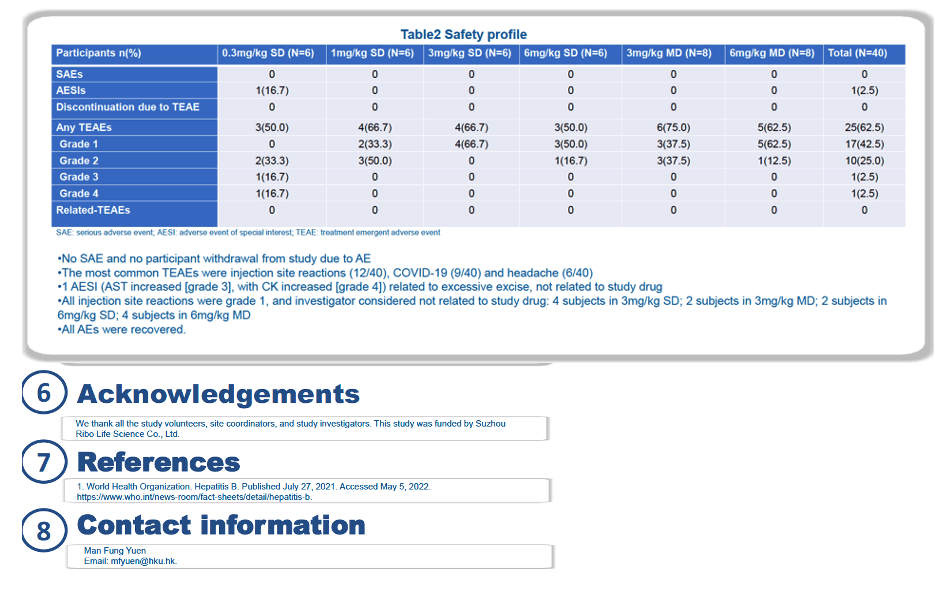
Through 24 weeks of treatment, researchers recorded no serious adverse events and no withdrawals from the study because of an adverse event. The only 2 grade 3 or 4 treatment-emergent adverse events (an aspartate aminotransferase jump to grade 3 and a creatine kinase elevation) occurred in people taking the lowest dose of RBD1016 and were judged not related to this siRNA.
Noting that RBD1016 inhibits HBV DNA replication and lowers levels of HBsAg in humans while promoting serological conversion in animal models, Ribo Life Science is developing the siRNA in hopes that it offers a functional cure for HBV infection [5].
References
1. Seto WK, Liang ZC, Gan LM, et al. Safety and antiviral activity of RBD1016, a RNAi therapeutic, in Chinese subjects with chronic hepatitis B virus (HBV) infection. EASL Congress 2023, June 21-24, Vienna. Abstract SAT-172.
2. Padda IS, Mahtani AU, Parmar M. Small interfering RNA (siRNA)-based therapy. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK580472/
3. ClinicalTrials.gov. A single and repeated dose escalation of RBD1016 in subjects with chronic hepatitis B virus (HBV) infection. ClinicalTrials.gov identifier NCT05017116. https://clinicaltrials.gov/ct2/show/NCT05017116?term=NCT05017116
4. Mak LY, Wong DK, Cheung KS, Seto WK, Lai CL, Yuen MF. Hepatitis B core-related antigen (HBcrAg): an emerging marker for chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2018;47:43-54. doi: 10.1111/apt.14376. https://onlinelibrary.wiley.com/doi/10.1111/apt.14376
5. Ribo news release. Ribo's siRNA drug RBD1016 for the treatment of chronic hepatitis B has completed a clinical study for safety and pharmacokinetic evaluation in healthy subjects. January 18, 2022. https://www.ribolia.com/En/newsroom-120

|
| |
|
 |
 |
|
|