| |
Bulevirtide+PegIFN HBsAg response
|
| |
| |
Download the PDF here
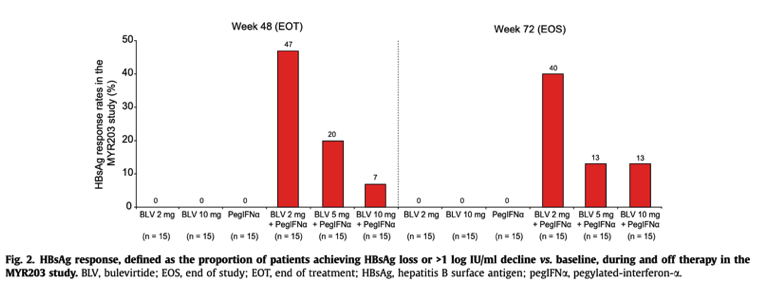
Ninety patients with chronic HBV/HDV co-infection were randomised into 6 treatment arms: pegIFNα 180 μg QW, 2 mg BLV+pegIFNα, 5 mg BLV+pegIFNα, 2 mg BLV, 10 mg BLV+pegIFNα and 10 mg BLV+TDF. In contrast to the MYR202 study, a nucleos(t)ide analogue backbone was not included in the first 5 treatment arms (Table S1). The primary efficacy endpoint, HDV RNA below the lower limit of detection (10 IU/ml) at week 72 (24 weeks off-therapy), was achieved by 0%, 53.3%, 26.7%, 6.7%, 6.7% and 33.3% of patients randomised to pegIFNα 180 μg QW, 2 mg BLV+pegIFNα, 5 mg BLV+pegIFNα, 2 mg BLV, 10 mg BLV+pegIFNα and 10 mg BLV+TDF, respectively. The corresponding ALT normalisation rates were 10%, 58.8%, 33.3%, 23.1%, 35.7% and 35.7%. HBsAg response defined as HBsAg loss or >1 log IU/ml decline at week 72 was observed only in patients treated with BLV combined with pegIFNα:40% (6 of 15 patients) for BLV 2 mg, 13.3% (2 of 15 patients) for BLV 5 mg, and 13.3% (2 of 15 patients) for BLV 10 mg (Fig. 2). HBsAg loss occurred in 4 (27%) and 1 (7%) patient(s) treated with BLV 2 mg or 10 mg plus pegIFNα, respectively. Combination therapy with pegIFNα showed strong synergism with respect to HDV RNA decline on treatment, but off-treatment HDV RNA responses at week 72 were only observed in patients achieving an HBsAg response.
|
|
| |
| |
|
|
|