| |
Postpartum Follow-up Care for Pregnant Persons With
Opioid Use Disorder and Hepatitis C Virus Infection
|
| |
| |
Challenges and barriers to completing the HCV clinical
care cascade - Download the PDF here
Postpartum Follow-up Care for Pregnant
Persons With Opioid Use Disorder and
Hepatitis C Virus Infection - Download the PDF here
Appendix 1. Flow chart of study inclusion criteria. -- Download the PDF here
May 2022 Jarlenski, Marian PhD, MPH; Chen, Qingwen MS; Ahrens, Katherine A. PhD, MPH; Allen, Lindsay PhD, MA; Austin, Anna E. PhD; Chappell, Catherine MD, MSc; Donohue, Julie M. PhD; Hammerslag, Lindsay PhD; Lanier, Paul PhD, MSW; McDuffie, Mary Joan MPH; Talbert, Jeffrey PhD; Tang, Lu PhD; Krans, Elizabeth E. MD, MSc, on behalf of the Medicaid Outcomes Distributed Research Network (MODRN)
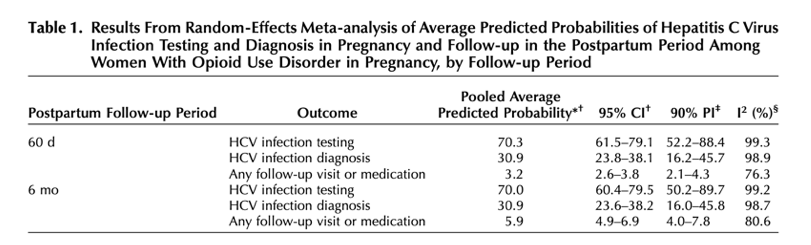
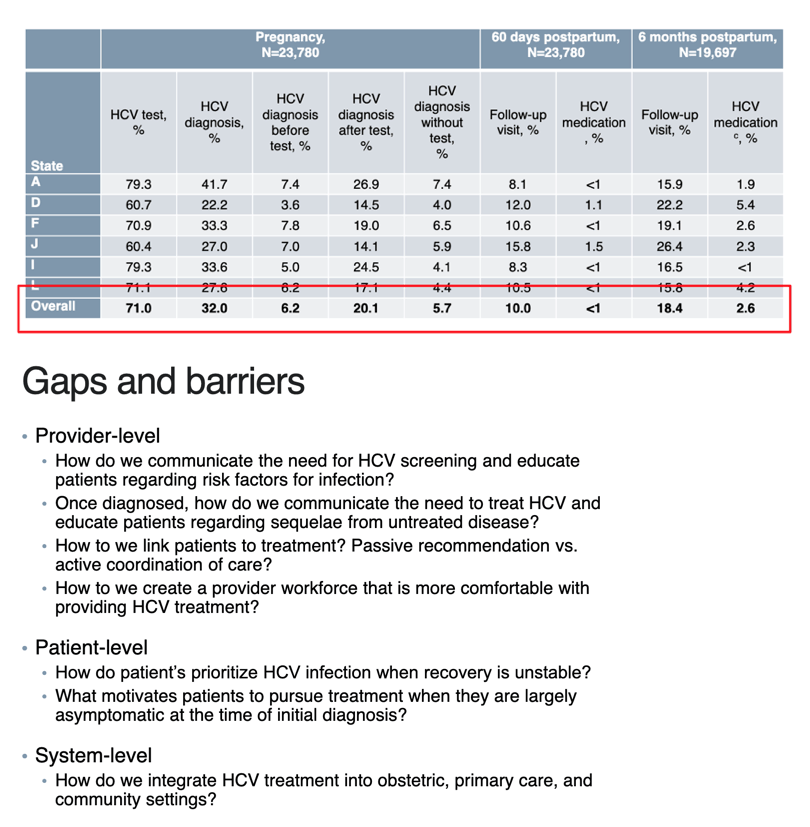
ONLY 70% were tested for HCV infection, 31% were diagnosed with HCV infection, and less than 6% had any follow-up visit or medication treatment, clearly inadequate & a failure.
In Brief
Among Medicaid-enrolled pregnant persons with opioid use disorder, one third are diagnosed with hepatitis C virus infection, but only 6% receive postpartum follow-up or medication treatment.
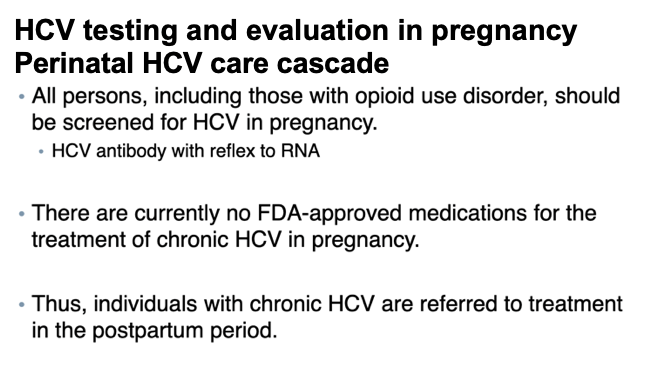
Hepatitis C virus (HCV) infections have risen alongside opioid use disorders (OUD) among people of reproductive age.1-3 Enhanced engagement with health care during pregnancy provides an opportunity for persons with OUD to access treatment for co-occurring disorders such as HCV infection.4 Guidelines recommend universal screening for HCV infection in pregnancy, with postpartum treatment for those with chronic HCV infection.5 Our objective was to estimate the prevalence of prenatal HCV infection testing and diagnosis and postpartum follow-up care among persons with OUD.
METHODS
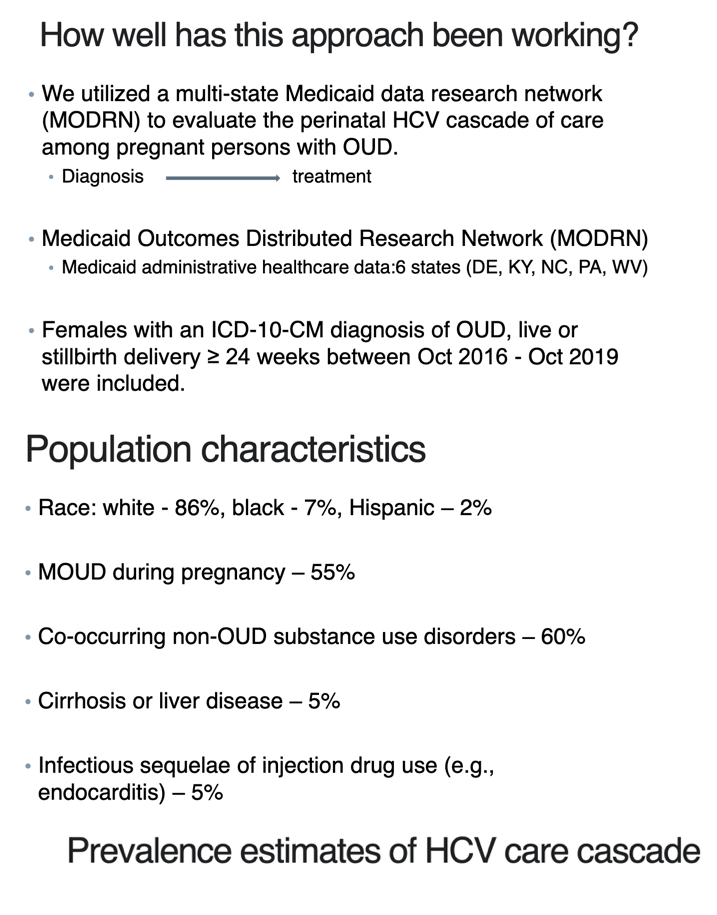
We conducted a retrospective cohort study using administrative data from six states in the Medicaid Outcomes Distributed Research Network: Delaware, Kentucky, Maine, North Carolina, Pennsylvania, and West Virginia. The Medicaid Outcomes Distributed Research Network common data model contains a census of enrollment, inpatient and outpatient utilization, and pharmacy records and provides reliable measurement across participating sites.6-8 We included 23,780 people who had a live birth or stillbirth between 2016 and 2019 and a diagnosis of OUD during pregnancy who were followed for 60 days postpartum; a subset of 19,697 (87%) people were followed for 6 months postpartum. This study was approved by site-specific institutional review boards: University of Delaware, University of Kentucky, University of Southern Maine, University of North Carolina at Chapel Hill, University of Pittsburgh, and West Virginia University.
Outcomes included binary measures of HCV antibody or RNA laboratory screening in pregnancy, diagnosis of chronic or acute HCV infection in pregnancy, and postpartum follow-up for HCV infection, defined as either an outpatient visit for HCV infection or medication treatment with a direct-acting antiviral.
Analyses controlled for demographic characteristics including age, race and ethnicity (non-Hispanic Black, non-Hispanic White, Hispanic, or none of these races), and urban compared with rural area of residence.9 Race and ethnicity are self-reported during Medicaid enrollment and are included as covariates because they may indicate differences in care due to structural or interpersonal racism.10 Clinical covariates included indicators for mental health conditions and substance use disorders, medication for OUD in pregnancy, sequelae of injection drug use, liver disease including cirrhosis, and anemia.
For each state, we fit three regression models in which the outcomes were HCV infection testing, HCV infection diagnosis, and postpartum HCV infection follow-up care. From these we derived average predicted probabilities and associated 95% CIs.11 To produce pooled estimates, we conducted a random-effects meta-analysis.12 We estimated between-state variability13 and calculated 90% prediction intervals, which provide a range in which estimates would be expected if we drew data from a different set of states. See Appendices 1-6, available online at https://links.lww.com/AOG/C664, for measurement and statistical details.
DISCUSSION
Among Medicaid-enrolled pregnant people with OUD in six states, 70% were tested for HCV infection, 31% were diagnosed with HCV infection, and less than 6% had any follow-up visit or medication treatment within 6 months postpartum. Although HCV screening and diagnosis rates varied across states, postpartum follow-up rates were low. Limitations include reliance on HCV infection diagnosis in medical records rather than detection of HCV RNA in the blood and lack of data on postpartum lactation, which could prevent direct-acting antiviral initiation. Our results suggest a need for improved postpartum HCV infection treatment (or antenatal treatment if safety is established)14 to realize the public health potential of universal screening in pregnancy.
RESULTS
The pooled average predicted probability of HCV infection testing during pregnancy among people with OUD was 70.3% (95% CI 61.5-79.1), with 90% prediction intervals indicating between-state heterogeneity (52.2-88.4) (Table 1). The average predicted probability of HCV infection diagnosis during pregnancy was 30.9% (95% CI 23.8-38.1), with 90% prediction intervals indicating heterogeneity (16.2-45.7). At 60 days postpartum, the average predicted probability of receiving any follow-up visit or medication for HCV infection was 3.2% (95% CI 2.6-3.8, 90% prediction interval 2.1-4.3); at 6 months postpartum, the average predicted probability of receiving any follow-up visit or medication for HCV infection was 5.9% (95% CI 4.9-6.9, 90% prediction interval 4.9-7.8).
------------------------------
Challenges and barriers to completing the HCV clinical
care cascade
Elizabeth E. Krans, MD, MSc
Associate Professor, University of Pittsburgh School of Medicine
Director, Perinatal Addiction Medicine, Department of Obstetrics, Gynecology and
Reproductive Sciences
Magee-Womens Research Institute
Full slide talk posted here.
Challenges and barriers to completing the HCV clinical
care cascade - Download the PDF here
HCV testing and evaluation in pregnancy
Perinatal HCV care cascade
71% received HCV test, 32% diagnosed with HCV - these are all women at high risk that should have 100% been screened
2.6% of high risk women with HCV were Treated in this study
| |
| |
| |
|
|
|