| |
New NASH Resmetirom Phase 3 Data at NASH-TAG Conference Jan 5
|
| |
| |
AASLD: A 52-Week Phase 3 Clinical Trial of Resmetirom in 180 Patients With Well-Compensated NASH Cirrhosis - (11/08/22)
AASLD: Utility of FIB-4, MRE, MRI-PDFF, & FibroScan to Identify Patients With At-risk F2-F3 NASH Based on Screening Data From a 2000 Patient Biopsy-Confirmed Cohort of Resmetirom Phase 3 Clinical Trial (MAESTRO-NASH) - (11/08/22)
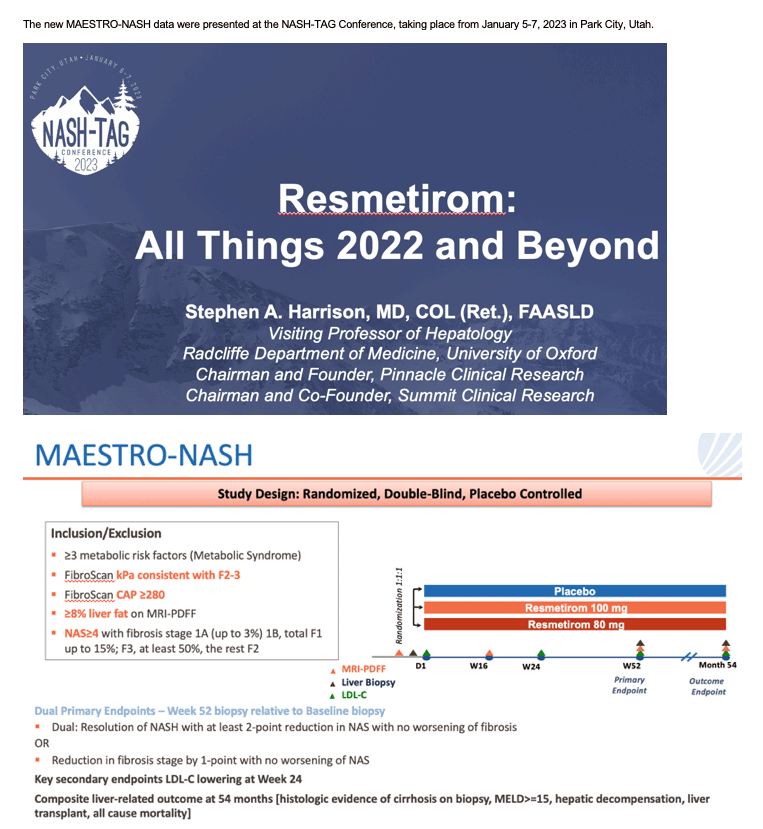
Dec 19 2023. Madrigal Announces Positive Topline Results from the Pivotal Phase 3 MAESTRO-NASH Clinical Trial of Resmetirom for the Treatment of NASH and Liver Fibrosis....https://ir.madrigalpharma.com/news-releases/news-release-details/madrigal-announces-positive-topline-results-pivotal-phase-3
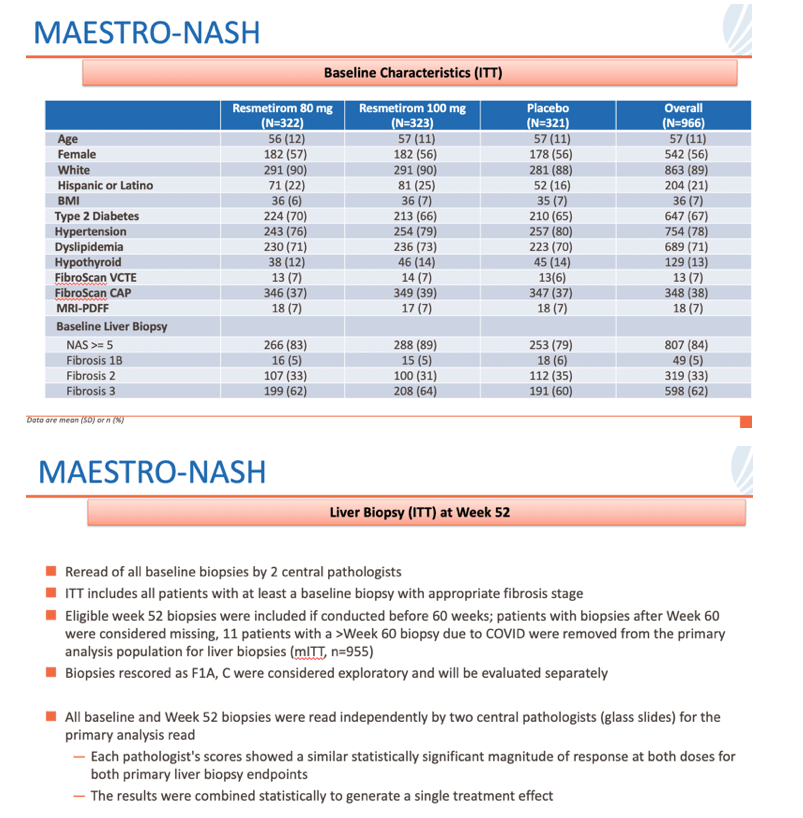
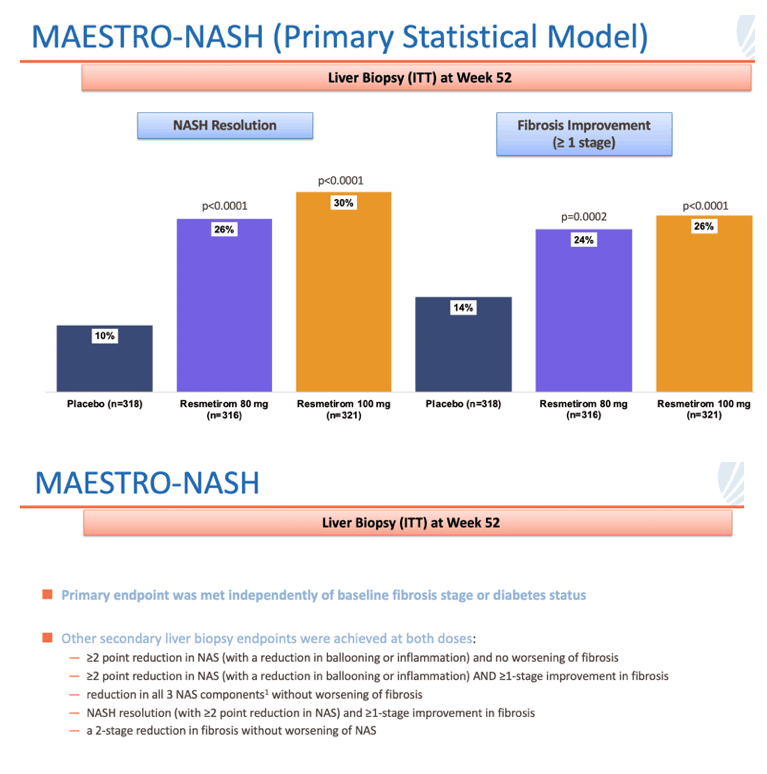
• In MAESTRO-NASH, a 52-week serial liver biopsy Phase 3 study in more than 950 patients, resmetirom achieved both primary endpoints and potentially clinically meaningful effects with both daily oral doses, 80 mg and 100 mg, relative to placebo
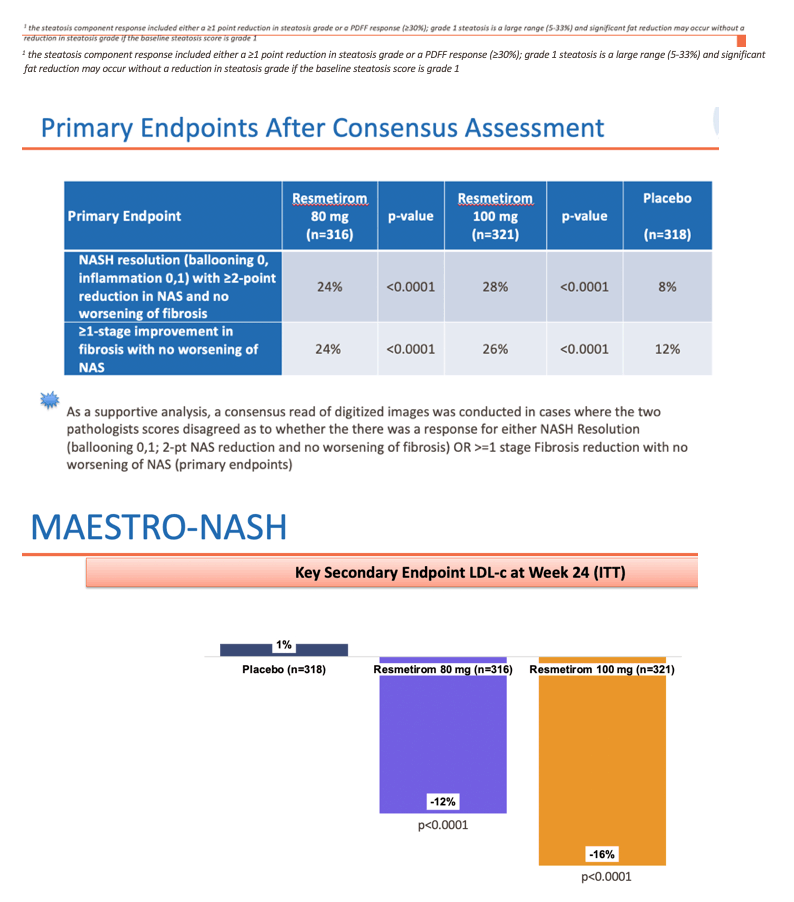
• NASH resolution (ballooning of 0, inflammation of 0-1) and ≥2-point NAS reduction with no worsening of fibrosis (p<0.0001 at both doses)
• Fibrosis improvement by at least one stage with no worsening of NAS (p=0.0002 and <0.0001 at 80 and 100 mg, respectively)
• Potentially clinically meaningful LDL-lowering, a key secondary endpoint (p<0.0001)
• Multiple positive effects on NASH biomarkers and imaging
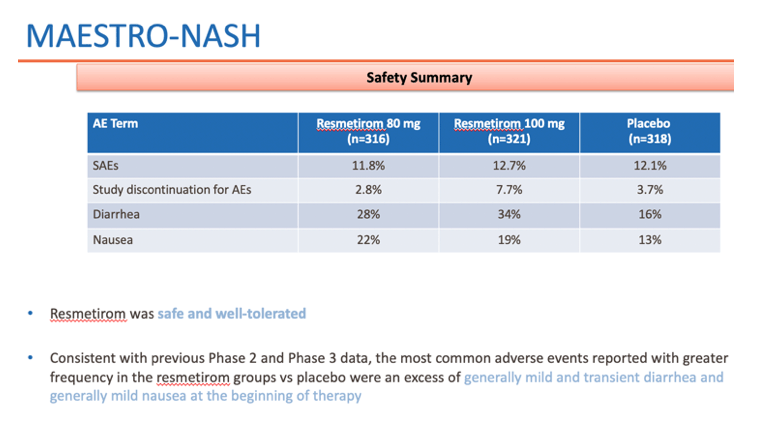
• Resmetirom was safe and well-tolerated in the MAESTRO-NASH study, consistent with the overall safety in Phase 3 MAESTRO trials, expanding the large safety database
• Madrigal intends to file a new drug application seeking accelerated approval of resmetirom for the treatment of non-cirrhotic NASH with liver fibrosis
Jan 6 2023. Madrigal Announces Additional Positive Results from the Pivotal Phase 3 MAESTRO-NASH Clinical Trial of Resmetirom for the Treatment of NASH with Liver Fibrosis
.....https://ir.madrigalpharma.com/news-releases/news-release-details/madrigal-announces-additional-positive-results-pivotal-phase-3
| |
| |
| |
|
|
|